 |
Watch Cells, Batteries, and Capacitors Explained Created 08-05/2002 This page attempts to explain, in layman's terms the difference between cells, batteries, and capacitors when used as a power source for quartz watches. |
| The Cell |
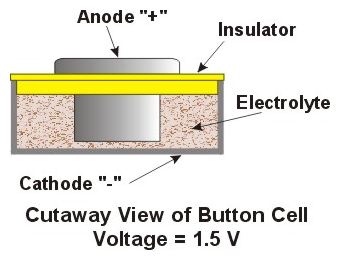 A cell is the smallest unit of electrical
storage power. A cell is the smallest unit of electrical
storage power.Cells consist of an assembly of 2 dissimilar metals in a chemical electrolyte. The 2 metals are referred to as the Anode (positive pole "+") and the Cathode (Negative pole "-"). The action of the electrolyte dissolving the Cathode creates electrical current which is used to power the watch. Once the Cathode is used up the cell is said to be "discharged" and is replaced with a new one.
|
| The Battery |
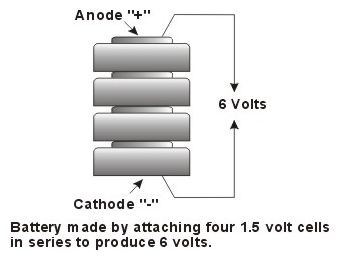 A battery is simply an assembly of 2 or more
cells connected in series to provide a voltage which is
the sum of the voltage of the cells. A battery is simply an assembly of 2 or more
cells connected in series to provide a voltage which is
the sum of the voltage of the cells.Batteries are seldom used in watches since most quartz watch movements operate on 1.5 volts (or less). The clue as to whether a
watch uses a cell or battery is in the voltage. Almost no watches use a battery. |
| The Capacitor |
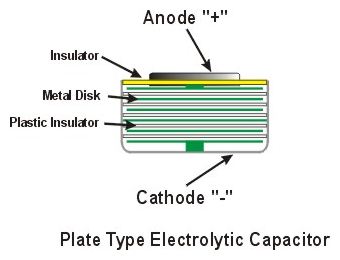 Shown at left is a cutaway of an
electrolytic capacitor of a type which could be used in a
watch. Shown at left is a cutaway of an
electrolytic capacitor of a type which could be used in a
watch.The capacitor differs from a cell or battery in that it can only store an electric charge applied to it. A capacitor does not produce electricity by chemical action. A large number of metal plates (green) are separated from each other by plastic insulators. Enough plates are used to produce the required voltage (usually 1.2 volts). The charging mechanism of the watch applies an electric charge to the plates which are capable of storing more energy than the watch uses in a certain amount of time. When the watch is not charging the capacitor the remaining charge inside the capacitor is used to power the watch.
The Seiko Kinetic line of watches uses a capacitor as a power source. |