|
Chemistry of Silicones
Oganosilicon
Compounds
Silicones

ˇ@
Organosilicon compounds
- Organosilicon compounds are those contain Si-C bonds.
The Si-C bonds are as strong as C-C bonds so silicon carbide SiC is extremely hared and
stable. Thousands of organosilicon compounds have been made artificially, most since 1950.
Many of these are inert and stable to heat (e.g. SiPh4 can be distilled in air
at 428 oC.)
By the Rochow 'Direct Process' Alkyl or
aryl halides react directly with a fluidized bed of silicon in the presence of large
amounts (10%) of a copper catalyst.
Si + 2CH4Cl à
(CH3)2SiCl2
(Cu catalyst 553-573K)
This is the main industrial method for making methyl
and phenyl chlorosilanes which are of considerable commercial importance in the production
of silicones. The yield is about 70%, with varying amounts of other products: MeSiCl3
(10%)and Me3SiCl (5%), and smaller amounts of Me4Si and SiCl4.
Both Grignard and direct methods yield a mixture of products, and very careful
fractionation is important as the boiling points are close.
ˇ@
- The silicones are a group of organosilicon polymers.
They have a wide variety of commercial uses as fluids, oils, rubbers and resins. Annual
production is estimated as about 300 000 tones. They are now produced on a larger scale
than any other group of organometallic compounds.
The complete hydrolysis of SiCl4
yields SiO2, which has a very stable three-dimensional structure. The
fundamental research of F.S.Kipping on the hydrolysis of alkyl substituted chlorosilanes
led to the discovery of long-chain polymers called silicones.
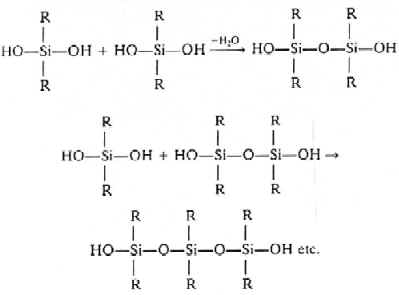
- The starting materials for the manufacture of
silicones are alkyl or aryl substitute chlorosilanes. Methyl compounds are mainly used,
though some phenyl derivatives are used as well. Hydrolysis of dimethyldichlorosilane (CH2)2SiCl2
gives rise to straight chain polymers and, as an active OH group is left at each end of
the chain, polymerization continues and the chain increases in length. (CH2)2SiCl2
is therefore a chain building unit. Normally, high polymers are obtained.
- ˇ@
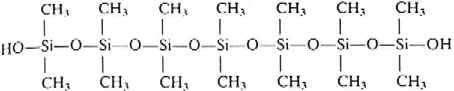
- Hydrolysis under carefully controlled conditions can
produce cyclic structures with rings containing three to six Si atoms.
-
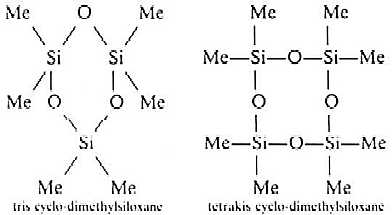
tris cyclo-dmethylsiloxane Tetrakis cyclo-dimethylsiloxane
- Hydrolysis of trimethylmonochlorosilane (CH3)3SiCl
yields trimethylsilanol (CH3)3SiOH, which is a volatile liquid. It
can condense and give hexamethyldi-siloxane. It cannot polymerize any more since there is
no OH groups.
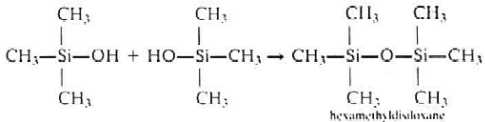
Hexamethyldisiloxane
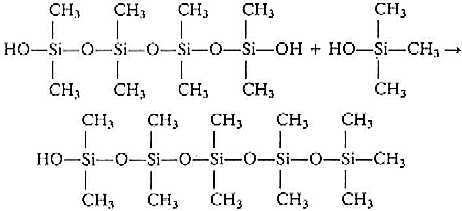
If some (CH3)3SiCl is mixed
with (CH3)2SiCl2 and hydrolysed, the (CH3)3SiCl
will block the end of the straight chain produced by (CH3)2SiCl2.
Since there is no longer a functional group at this end of the chain, it cannot grow any
more at this end. Eventually the other end will be blocked in the similar way. Thus (CH3)3SiCl
is a chain stopping unit, and the ratio of (CH3)3SiCl and (CH3)2SiCl2
in the starting mixture will determine the average chain size.
ˇ@
Silicones are comparably economic while
with many desirable features. They were originally developed as electrical insulators,
because they are more stable to heat than organic polymers. Also when they break
down, no conducting materials will be released as carbon does. So they are good electrical
insulators.
Their water repellency is due to being surrounded by
organic side groups, which give them the low surface tension. Silicones may exist as
liquids, oils, greases, rubbers or resins. Straight chain polymers of 20 to 500 units form
silicone fluids. They make up 63% of total silicones uses. When the length of chains
increase, the viscosity and boiling point will increase as well. Then different products
can be produced by controlling the chain length.
Silicone rubbers are made of long straight chain
polymers between 6000 and 600,000 Si units long. They are useful because they can retain
their elasticity from 183K-533K. If cross-links are formed, the polymers will become
brittle and cracks, and low molecular weight, cyclic structures are evolved. Strong
heating in the absence of air causes silicones to soften and form volatile products.
Silicone resins are rigid polymers. They are
extensively cross-linked. About 12% of silicones produced are resins.
Although organosilicon compounds are
very useful, they cannot replicate organic compounds because:
 | Silicon has little tendency to bond itself, while
carbon has a strong tendency to do so. |
 | Silicon does not form pŁk-pŁkdouble
bonds, which forms readily in organic compounds. |
 | Silcon forms a number of compounds containing pŁk-dŁkdouble
bonds in which the silicon atom uses d orbitals. |
- ˇ@
ˇ@
Back to top
|