
Monosaccharides
A monosaccharide is a carbohydrate that can't be hydrolyzed to simpler carbohydrate units. The monosaccharide is the basic carbohydrate unit of cellular metabolism.
Monosaccharides are single sugar units. Their general formula is (CH2O)n. They are classified according to the number of carbon atoms as trioses (3C), tetroses (4C), pentoses (5C), hexoses (6C) and heptoses (7C). Of these, pentoses and especially hexoses (e.g. glucose, fructose, galactose) are the most common so we will concentrate on them.
The hexose monosaccharides are the most important carbohydrate sources of cellular energy. 3 hexoses ¡V glucose, galactose, and fructose ¡V are of major significance in nitrition. All 3 have the same molecular formula and thus contain an equal no. of reduced carbons. They differ in structure but are biologically interconvertible. Glucose plays a cenral role in carbohydrate energy utilization. Other carbohydartes are usually converted to glucose before cellular utilization.
Glucose is the most important of the monosaccharides. It is an aldohexose and is found in the free state in plant and animal tissue.
Structure

Open chain and ring forms
Structure of the open chain and a and b ring forms of glucose. The three forms exist in equilibrium in aqueous solution, with 0.02 % open chain, 36% a glucose and 64% b glucose.
The above graph shows glucose as having either an ¡¥open chain¡¦ or ring structure. The open chain form can be straight, but because of the bong angles between carbon atoms it is possible for sugars with 5 and 6 carbon atoms to bend and form stable ring structures. In hexoses like glucose, the first carbon atom combines with the oxygen atom on carbon atom no. 5 to give a 6-membered ring. Oxygen is part of the ring and that 1 carbon atom, carbon atom no. 6, sticks up out of the ring. In pentoses, the 1st carbon atom joins with the oxygen atom on the 4th carbon atom to give a 5-membered ring.
The ring structures of pentoses and hexoses are the usual forms, with only a small proportion of the molecules existing in the open chain form at any 1 time. The ring structure is the form used to make dissaccharides and poly saccharides.

Alpha and beta isomers
The graph has also showed that glucose can exist in 2 possible forms, known as the alpha and beta forms. The hydroxyl group on carbon 1 can project below the ring (a glucose) or above the ring (b glucose). Molecules like this, which have the same chemical formula but with different structures are said to be the isomers of each other. At any given moment in a glucose solution, some of the molecules will be in the open chain form (0.02%) and some in the ring form. This is more stable and therefore more common. A glucose molecule can switch spontaneously from the open chain form to either of the2 ring forms and back again. Overall equilibrium is reached where the proportions of the different forms remain constant.
As stated above, only the ring form can be used to make disaccharides and polysaccharides. Despite the relatively small difference in structure between a and b glucose, there are important consequences. a glucose is used to make the polysaccharide starch and b glucose the polysaccharide cellulose molecules which have very different properties.
Any 2 monosaccharides that differ only in the configuration around a single carbon atom are called epimers. Thus D- and L-glyceraldehyde are epimers.
The structure called D-glucose is so named because the ¡VH and ¡VOH on carbon 5 are in the same configuration as the ¡VH and ¡VOH on carbon 2 in D-glyceraldehyde. The configuration of the ¡VH and ¡VOH on carbon 5 in L-glucose corresponds to the ¡VH and ¡VOH on carbon 2 in L-glyceraldehyde.
Function
Glucose is commonly known as dextrose or grape sugar. It is a component of the disaccharides sucrose, maltose, and lactose, and is also the monomer of the polysaccharides starch, cellulose and glycogen. Among the common sugars, glucose is of intermediate sweetness.
It is the most common respiratory substrate & the most common monosaccharides. It is also the key sugar of the body and is carried by the bloodstream to all body parts. The concentration of glucose in the blood is normally 80-100 mg per 100ml of blood. Because glucose is the most abundant carbohydrate in the blood, it is also sometimes known as blood suar. Glucose requires no digestion and therefore may be given intravenously to patients who cannot take food by mouth. Glucose is found in the urine of those who have diabetes mellitus (sugar diabetes). The condition in which glucose is excreted in the urine is called glycosuria.
Fructose, also known as levulose, is a ketohexose that occurs in fruit juices, honey, and (along with glucose) as a constituent of sucrose.
Structure
The open chain form can be represented in a Fischer projection formula:

Like glucose and galatose, fructose exists in both cyclic and open chain forms. One common cyclic structure is a five membered furanose ring in the b configuration:

Function
Fructose is the major constituent of the polysaccharide insulin, a starchlike substance present in many plants. It is the sweetest of all the sugars,being about twice as sweet as glucose. This accounts for the sweetness of honey. The enzyme invertase, present in bees, spl;its sucrose into glucose and fructose. Fructose is metabolized directly but is also readily converted to glucose in the liver.
Galactose is also an aldohexose and occurs, along with glucose, in lactose and in many oligo- and polysaccharides such as pectin, gums, and mucilages. Galactose is an isomer of glucose, differing only in the spatial arrangement of the ¡VH and ¡VOH groups around carbon 4.
Structure
Galatose, like glucose, is an aldohexose and differs structurally from glucose only in the configuration of the ¡VH and ¡VOH group on the 4th carbon:
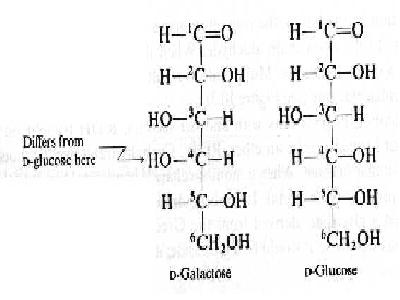
Galatose, like glucose, also exists primarily in 2 cyclic pyranose forms that have hemiacetal structures and undergo mutarotation:
Function
Galactose is synthesized in the mammary glands to make the lactose of milk. It is also a constituent of glycolipids and glycoproteins in many cell membranes such as those in nervous tissue. Galactose is less than half as sweet as glucose.
4.Chief function of monosaccharides
| ¡@ | Examples | Functions |
| Trioses |
|
|
| Pentoses |
|
|
| Hexoses |
|
|