Kovalenko R.I., Sibarov D.A., Nozdrachev A.D.,
The influence of unilateral olfactory epithelium stimulation on rat pineal cells during the stress.
Advances in Comparative Endocrinology, Proceedings if the 13th International Congress of Comparative Endocrinology, Yokohama, Japan, 1997, p.697-700.
Department of Human and Animal Physiology, St.-Petersburg State University, St.-Petersburg, Russia
SUMMARY
Electrophysiological and electron-microscopy study of intact and stress-subjected male Wistar rat pineal cells were performed. The increase of pinealocytes secretory activity was shown in stress. Furthermore, the suppressory effect of unilat eral oxytocin intranasal infusions or electric stimulation of olfactory epithelium was found.
INTRODUCTION
Pineal gland (PG) is known to play the important role in adaptive reactions forming on variable conditions of environment. Melatonin and pineal peptide factors are involved in reactions on osmotic (Kovalenko, 1993), hypoxic (Galantsev et al. , 1995) influences, initial stress attack (Milin, 1984) and so on. Pineal interactions with visual system are well discribed (Kovalenko, 1993; Chernysheva, 1995; Reuss, 1996). But it’s links with other sensory organs was a subject of few works. In paticu lar anosmia (made with ZnSO4 destruction of nasal mucosa) was reported to increase pineal functional activity and growth of blood melatonin like in the case of blinded animals (Reiter, 1991). Evoked potentials of pinealocytes were shown while habenular nuclei stimulation (Ronnekleiv et al., 1980; Semm et al., 1981). The latter is known to be the part of a pathway between olfactory epithelium, limbic structures and PG. An asymmetry of central and peripheral effects was noticed while inilateral intranasal injections of oxytocin (OT) and pineal neuropeptides (Nozdrachev et al., 1994; Kovalenko et al., 1995). An activation of left olfactory bulb caused greater sympathetic influence on visceral organs and muscles, while the right - parsympathetic (Nozdrachev et al., 1992). This asymmetry was better exhibited during the stress. Data obtained led us to suppose that the asymmetry of effects during inilateral olfactory epithelium stimulation involves olso PG. Elaboration of this hypothesis was an ai m of this wotk.
MATHERIALS AND METHODS
20 adult males of Wistar rats (180-240g) kept in natural day-night rhythm were used in this study. Experiments were performed in the day-time 13:00 - 15:00. Intact animals which had a free access to food and water served as c controle. Rats of experimental group were stress subjected (48-hour water and food deprivation). Some of deprived rats (N=6) were inilaterally intranasally infused with OT solution (1.5´10-17M in 15 m/FONTl 0.9% NaCl). Aft er 15 minutes in open feeld this rats and intact (N=4) were decapitated and PG was elicited. Pineal tissue was fixated in 2% glutaraldehyde in K+/Na+-phosphate buffer (0.12M, pH 7.3), washed in buffer, gradually dehydrated in alcoho le, aceton and then embeddled in EPON. Sections were made on LKB-III ultratome, contrasted with 1,5% uranil acetate and lead citrate. Survey and photography was performed on Hitachi-300 microscope in transmission mode. Ultrastructural features of pineal secretory activity were studied. Noticed changes occured in >80% cells. Some deprived (N=5) and intact (N=5) rats, were urethane-anesthetized (1,1g/kg body weight i.p.), fixed in Page 2 stereotaxic frame in screened grounded camera. Dorsal surface of the brain was exposed by craniotomy with a special milling cutter. Saggital sinus was ligated and cut. Glass microelectrodes with a tip diameter of 10- 30 m m (resistance 4-6MW ) filled with 3Ì NaCl was visually positioned at dorsal surface of the PG. To increase the signal amplitude nistatin (Squibb & Sons Ltd.), producing artificial ion channels in cell membrane, was added to electrode solu tion (Inushin, 1996). Extracellular action potentials were recorded from the superficial part of the PG, and stored on FM-tape for off-line computer analysis. Parameters of single cells and intercellular interactions were investigated (>150 cells stud ied). For stimulation experiments bipolar silver electrode was used for unilateral stimulation of olfactory epithelium (40 V, 100Hz, 50 ms during 15s). Results were statistically analysed useing ANOVA, spectral and autocerrelation analysis.
RESULTS AND CONCLUSIONS
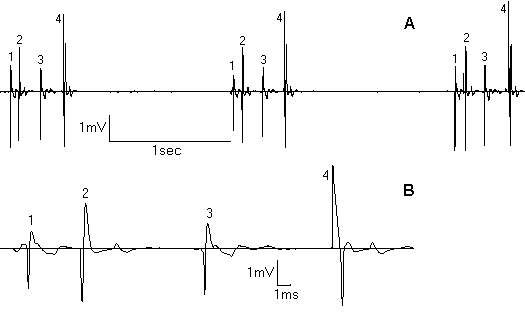
Electron-microscopy study demonstrated the prediminance of light pinealocytes in PG parenchyma in comprasion to dark cells in all groups of rats. 48-hour water and food deprivation lead to an activation of secretory processes in pinealocytes (secretory vesicles accumulation in the cell body and processes, their exocytosis, decondensation of nuclear chromatin, Golgi complex hypertrophy). It was accompanied with mitochondrial structural damage. In dark pinealocytes there were no such changes. Electrophysiological study revealed two types of cellular activity: “slow” (<2Hz) and “fast” (>4Hz). Only “slow” type of activity (0.92±0.49Hz) was usually registered in intact animals. In stress, frequency of “slow” component increased 4-6 tymes thanks to conversion to phasic type of activity (tab. 1). “Fast” component also appeared (5.63±1.06Hz). As a result, electron-mycroscopy study demonstrating an activation of pineal secretion during the stress were confirmed with electrophysiological data . We suppose the registered activity to be produced by light pinealocytes. Other authors (Reuss, 1986; Stehle et al., 1987; Parkington et al., 1987) also discribed regular, irregular and phasic pinealocytes activity. In stress-subjected animals groups of interacting pineal cells were noticed (fig. 1A). Pattern of such a group looks like phasic, but action potentials of this cells are different and organised in strict consequence with unqual delays between spikes (fig. 1B). This cells interaction probabl y realises an activation of a group of pinealocytes by single nerve terminal. Unilateral intranasal OT infusions caused the suppression of light pinealocytes secretory processes. For left-side OT infusion it was inhibited extrusion, for right-side it was inhibited synthesis of secretory material (a few dence cored vesicles, condenced nuclear chromatin). The signs mitochondrial damage and Golgi complex hypertrophy noticed in not infused rats disappeared. The decrease of PG secretory activity while OT inf luence on olfactory epithelium was confirmed with electrophysiological investigation, where it’s inilateral electric stimulation caused significant decrease of summar frequency of spikes by 58% in deprived and by 45% in intact rats (P<0.05). The “slow ” component was mainly affected. Thus Page 3 noticed suppression of PG secretory activity while olfactory epithelium stimulation tells us about pineal neural links with one more sensory system - olfactory.
REFERECES
Chernysheva M.P., Animal hormones St.Petersb., Glagol, 1995.
Galantsev V.P., R.I.Kovalenko et al., Pineal peptides influence on tolerance and tissue lipid peroxydation systems in rats during asphyxia and reoxygenation (russian), Vestnik SPbGU, ser.3, 3(17), 68-77, 1995.
Inushin M.U., Tzitzarev V.U. et al., The use of artifitial ion channels for semiintracellular registration of brain neurons activity., Sechenov Physiol.J., 82(1), 1996, 18-24.
Kovalenko R.I. The pineal gland (russian) Neuroendocrinology, part.1, book.2, SPb. RAN, 300-323, 1993.
Kovalenko R.I., Chernysheva M.P., Shtylik A.V., Nozdrachev A.D., An asymmetry of peripheral effects of unilateral oxytocin infusions in rat (russian), Dokl.RAN., 342(2), 269-272, 1995.
Milin J., Martinovic J., Demajo M., Morphodynamic responce of the pineal gland to initial stress attack, Arch.d’Anat.micr., 73(3), 1984, 159-180.
Nozdrachev A.D., Osipova N.S., Chernysheva M.P., Oxytocin role in rostral brain structures asymmetry during the stress (russian), Sechenov Physiol.J., 78(2), 269-272, 1992.
Nozdrachev A.D., M.P.Chernysheva et al., Oxytocin’s role in rostral brain structures asymmetry during the stress (russian), Sechenov Physiol.J., 80(6), 1994.
Parkington H.C., McCance I., Coleman H.A., Two types of cells with central innervation in pineal gland of guinea pigs, Am.J.Physiol, 252, 369-377, 1987.
Reiter R.J. Pineal melatonin: cell biology of it's synthesis and of it's biological interaction, Endocr. Rev., 12, 151-180, 1991.
Reuss S. Conponents and connections of circadian timing system in mammals, Cell Tiss.Res., 285, 353-378, 1996.
Reuss S., Vollrath L. Electrophysiological properties of rat pinealocytes: evidence for circadian and ultradian rhythms, Exp. Brain Res., 3, 87-94, 1986.
Ronnekleiv O.K., Kelly M.J., Wurrke. W., Single unit recordings in the rat pineal gland: evidence for habenular-pineal neural connections, Exp.Brain Res., 39, 187-192, 1980.
Semm P., Schneider T., Vollrath L., Morphological and electrophysiological evidence for habenular influence on the guinea-pig pineal gland, J.Neur.Transm., 50, 247-266, 1981.
Stehle J., Reuss S., Volranth L. Electrophysiological characterisation of the pinela gland of golden hamsters, Exp.Brain Res., 67, 27-32, 1987.