Sibarov D.A.,Kovalenko R.I.,Anisimov V.N.,Nozdrachev A.D.,
Daytime pineal gland activation in rats with colon tumors induced by 1,2-dimethylhydrazine
Neuroendocr.Lett., 2000, 21: 307-312.
General Physiology Dep., St.-Petersburg State University.
N.N.Petrov Research Institute of Oncology
OBJECTIVES: Intact rats and rats with 1,2-dimethylhydrazine induced tumors of large intestine were used in experiments. Previously, blood melatonin concentration in that tumor-bearing rats was shown to increase at night, but not in the daytime.
METHODS: The extracellular microelectrode registration of rat day-time pineal glands activity was performed.
RESULTS: The existence of different types of pinealocytes in the pineal gland was confirmed. Tumor-bearing rats, in comparison to intact, demonstrated higher spikes frequency due to cells switching from regular to pattern (4-6 times gain) activity and appearance of “fast” cells (>5Hz frequency).
CONSLUSIONS: The literature about pinealocytes points on the correlation between electrical and secretory processes in pinealocytes, thus we suppose the groups of interacting cells, detected in tumor-bearing rats, to reflect cascade cells activation w hile pineal gland secretion increase. The results indicates, that in the day-time pinealocytes are secreting substances (not melatonin) in dependence with hormonal background.
Key words: pinealocytes, colon tumor, 1,2-dimethylhydrazine, electrical activity, secretion, melatonin
INTRODUCTION
As photoneuroendocrine transducer the pineal gland modulates many of physiological systems activity according to circadian rhythms. It is a part of the system controlling adaptive reactions of organism on various conditions of environment [27,28]. Pi neal indolamine and peptide hormones influence immune functions [5,7,18]. Melatonin, in particular, increases immune memory while T-dependent antigene immunization and stimulates the antibody production. Pinealectomy or constant light regime, which suppr esses pineal activity, promotes tumor processes, whereas melatonin injections resulted in the decrease of carcinogenesis [2,3,4,6]. Many investigators attach too much importance to melatonin, ignoring the other pineal hormones and pineal function in the day-time. But except melatonin, the pineal gland produces the multitude of peptides. This peptides have a wide range of activity. In particular, they are able to suppress RNA synthesis in tumor cells [22] and to selectively modulate DNA transcription [21].
Microelectrode registration of action potentials, which are correlated with exocytosis in neurosecretory cells [9,10,20], allows us to estimate exocytosis intensity in pinealocytes. Active night-time pineal melatonin secretion and electrical activity are usually supposed to be unquestionably connected. But, in the day-time pineal melatonin production is known to be low, thus the day-time electric activity op the pineal gland is doesn't reflect it's secretion. Melatonin is a highly lipophilic substanc e and can pass the cell membrane freely, thus it's release is probably not connected with the excretion of secretory vesicles, and, consequently, not connected with the cellular membrane electrical processes. It can be confirmed with the following obser vations. Elimination of action potential-like spikes with exposure to Ca2+-binding substances like verapamil or nifedipine or removal of extracellular calcium, did not substantially affect melatonin production [19]. But, the pineal peptide and serotonin secretion seems to be excreted with an ordinary exocytosis, which in neurosecretory cells is usually accompanied with membrane depolarisation [9,10,20]. Furthermore, previously we have noticed the correlation between day-time pinealocytes secr etion (an accumulation of secretory vesicles with peptide content) and their electrical activity [12].
The aim of this work was to corroborate the hypothesis about involving the pineal gland in inhibition of tumor progression. Secretory activity of pineal cells was estimated according to their electrical activity in intact animals and in tumor-bearing rats.
METHODS
Wistar rats, breeded in N.N.Petrov Research Institute of Oncology (St.Petersburg) were used in experiments. The study was performed according to FELASA guidelines (Category C). 2-3 month aged rats were weekly 5 times subcutaneously injected wit h 1,2-dimethylhydrazine dihydrochloride (DMH, Sigma, USA) in dose (calculated to base) of 21mg/kg or 0,5ml 0,9% NaCl for intacts. Solutions were made ex tempore and neutralized with sodium bicarbonate (ðÍ = 7.0). 4-6 m onth later such DMH dose inevitably caused adenocarcinoma in the large intestine. Rats were kept in 12:12 day/night rhythm, fed with standard laboratory fodder and had a free access to water.
4 month after the last DMH injection, 5 injected and 5 intact rats were used for electrophysiological investigation. Experiments were performed in day time from 13:00 to 15:00, because pineal melatonin production is known to be lower in the day-time. We suppose, that it would be easier to detect an activation of peptide secretion on such background. Urethane-anaesthetised rats (1,2 g/kg body weight, i.p.) were fixed in stereotaxic frame in screened and grounded camera. Dorsal surface of the brain wa s exposed by craniotomy with a special milling cutter. Saggital sinus was ligated and cut. Glass microelectrodes with a tip diameter of 10-30 m m (resistance 4-6MW ) filled with 3Ì NaCl were visually positioned at dorsal surface of the pineal gland. Action potentials were extracellulary recorded from the superficial part of the pineal gland, and stored on FM-tape for off-line com puter analysis. Parameters of single cells and intercellular interactions were investigated (>160 cells studied).
Extracellular microelectrode registration allows to simultaneously record discharging of 1-10 cells. We have developed the software for analysing such records and for extracting the "voices" of separate cells. For this purpose an amplitude, length of depolarisation and repolarization and polarity were measured for each spike; regular and pattern forming groups of spikes were sought for. The search of interacting cells was performed. The basic principles of this analysis [8] are widely used by many au thors for investigation of neuronal structures.
After electrophysiological investigation the intestine was taken off and prepared to measure tumor square. Correlation between medium frequency of pineal cells discharging and tumor square was calculated.
RESULTS AND DISCUSSION
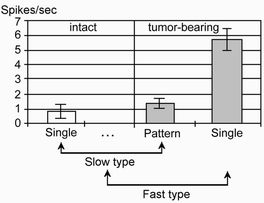
“Slow”, with spikes frequency < 2Hz, and “fast”, with spikes frequency >4Hz, type of pineal cells activity were revealed in this study. “Slow” type of activity were presented by regular, irregular and pattern spikes (Fig.1). Electrophysi ological parameters of pinealocytes activity in intact animals and in tumor induced rats are presented in Table.1. Intact rats usually demonstrated only “slow” type of activity (frequency 0.86±0.49Hz, regularly and irregularly discharging cells).
Data obtained allows us to suppose comparatively low pineal peptide secretory activity in intact rats. Previously the same conclusion about day-time low pineal secretion of intact animals was made during cells utrastructural study with electron-micros copy, where we have observed insignificant number of vesicles of different nature in pinealocytes of intact as compared to osmotic stress-subjected rats where a lot of peptide-containing vesicles were present in cytoplasm and in cell processes [13].
In colon tumor-bearing animals pineal cells with pattern activity were revealed. This type of activity was absent in intact rats. In tumor-bearing rats “slow” cells increased spikes frequency 4-6 times due to switching to pattern discharging, “fast” t ype of activity also appeared (5.71±0.73Hz) (Fig.2).
Pattern activity was supposed to appear in regularly discharging cells during their secretory activation. This assumption was confirmed with the following observations:
1) reversible switching between regular and pattern discharging in one cell;
2) variable number of spikes in a pattern;
3) frequency of patterns and frequency of regularly discharging cells are close and belong to “slow” type of activity;
4) longitude of single spike coincides in “pattern” and in “regular” cells.
The results of our electrophysiological study of intact and colon tumor-bearing animals are concordant with the existence of several cellular types in the pineal gland [26]. It is interesting, because for a long period only two type of pineal cells - “light” and “dark” were supposed to be presented in the gland. Recently several researchers [11] have identified more types of pineal cells namely light, dark, intermediate and granular in foxes. Besides, the cells were decided to belong to a certain typ e according to their current functional state, because cell optical density depended of time. Pinealocytes with regular, irregular and pattern activity were electrophysiologically identified by different authors [23,25,26,30]. They have established, tha t spike longitude in different cell types vary from 1ms to about 200ms. Diversity in types of cellular activity and in spikes parameters helped us to distinguish separate cells in multicellular recordings.
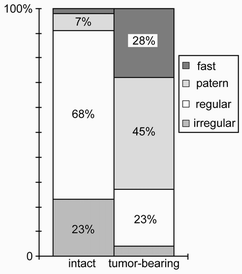
The ratio of cells with different types of activity were distinguished in colon tumor-bearing and in intact rats (Fig.3). The main part of cells in intact rats (91%) were presented by “regularly” and “irregularly” active cells, thus explaining the lo w summary frequency of spikes. In tumor-bearing rats the number of “pattern” and “fast” cells increased and consequently increased the summary frequency. 3-times decrease of “regular” cells number and simultaneous 550% increase of “pattern” cells number also points on switching of cells to pattern type of activity. This fact and the decrease of “irregular” cells number and increase of “fast” cells number reflects intensive pineal secretory processes in rats with tumors in large intestine.
In previous experiments [4] DMH-tumor induced rats demonstrated higher serum melatonin concentration only at night, but not in the day-time, as compared to intact animals. Colon tumors progression may decrease the number of melatonin secreting enteroc hromaffine cells [15], which decreases reduces the protection of organism against tumor. Pineal hormones (both melatonin and peptides) are known to have antitumor properties. The lack of melatonin leads to compensatory increase pineal melatonin (night-ti me) and peptide (day-time) production. On the basis of known correlation between electric end secretory activity of neurosecretory cells [9,10,20] we have supposed the higher pineal electric activity in the day-time (not at night) to reflect intensive no n-melatonin secretion. We think that day-time activation of pineal electric activity in DMH-treated rats reflects secretion of peptides, because in our previous study [12,13] the higher frequency of pinealocytes depolarisation was accompanied by an accu mulation and exocytosis of peptide/protein containing vesicles in osmotic stress-subjected rats. The ratio of different cellular types, medium frequency of spikes and other peculiarities of electrical activity in tumor-bearing rats looked similar to the same parameters in 48-hour water and food deprived rats [12].
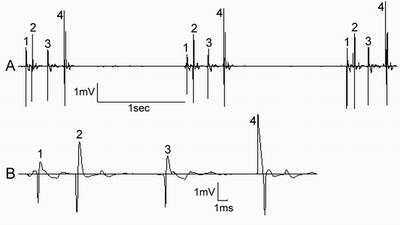
The groups of interacting cells were noticed in colon tumor-bearing rats (fig.4À). It looked like pattern activity, but cells could be distinguished by spikes form, and cells were discharging in strict consequence with d ifferent delays between spikes (fig.4B). Such intercellular interactions, probably, realize an activation and synchronic discharging of groups of cells due to existence of tight [14] and gap junctions [29], which probably transfuse signals betwee n the pinealocytes.
An increase of pineal electric activity during tumor process in the large intestine may be also due to an activation of sympathetic system. Such activation takes place, for example, in stress [17,24,28]. So, we suppose the higher electrical activity o f pinealocytes, which in the day-time reflects non-melatonin excretion, and higher blood melatonin concentration in the night, detected by us, to be adaptive reaction against tumor forming, because both melatonin [1,2,3,6] and pineal peptides [3,16,22], are known to have anticarcinogenic properties.
In our experiments tumors in the large intestine were revealed in all DMH-treated rats. The square of tumors was 56 ± 32 mm2 , but there were no correlation between the square and the frequency of spikes of spontaneously pineal active cells.
Thus, the results of our investigation demonstrated that pinealocytes of tumor-bearing rats are secretory active not only at night, but also in the day-time, as compared to intact rats. Tumor presence or humoral disorders while 48-hour water and food deprivation have a similar incentive effect on pineal secretory processes even in day-time.
LITERATURE CITED
Table 1. Comprasion of different type pineal cells in intact and tumor-bearing rats
| The type of activity |
Intact rats |
Tumor-bearing rats |
| “Slow type” (frequency of spikes< 2Hz) |
regular single spikes (0.86±0.49Hz) and irregular spikes |
regular pattern activity (1.43±0.35 patterns/sec; 4-6 spikes in a pattern) and regular single spikes |
| “Fast type” (frequency of spikes > 4Hz) |
usually absent |
regular spikes (5.71±0.73Hz), sometimes short patterns (2-3 spikes in a pattern). |
FIGURE LEGENDS
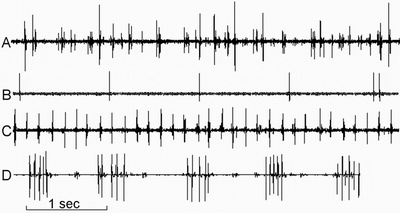
Fig. 1. The examples of spontaneously active pineal cells:
A - multicellular activity;
B - “slow” type cell in intact rat (regular spikes);
C - “fast” type cell in colon tumor-bearing rat (regular spikes);
D - “slow” type patterns in colon tumor-bearing rat cell.
Fig.2 Spontaneously active pineal cells frequency comprasion in intact and colon tumor-bearing rats.
“Slow” type regulary discharging cells of intact rats are compared with “slow” type pattern activity cells of colon tumor-bearing rats. It allows us to show that summary frequency increased 4-6 times bacause of switching of cells from “regular” to “pattern” type of activity (from 1 spike to 4-6 spikes in a pattern).Fig 3. The ratio of different cell types in intact and colon tumor-bearing rats Fig. 4. The group of 4 interacting pineal cells in colon tumor-bearing rat:
A - constant intervals 1-2, 2-3, 3-4 and 4-1 between spikes,
B - the different spikes amplitude, form and polarity. Unequal intervals 1-2, 2-3, 3-4.