Sibarov D.A., Kovalenko R.I., Malinin V.V., Khavinson V.Kh.
EPITALON INFLUENCES PINEAL SECRETION IN STRESS-EXPOSED RATS IN THE DAYTIME
Neuroendocr. Lett., 2002, Dec., 23(5/6): 473-475.
Acad. I.P. Pavlov Institute of Physiology of the Russian Academy of Sciences St.
Petersburg State University, Department of General Physiology
St. Petersburg Institute of Bioregulation and Gerontology of the North-Western Branch of the Russian Academy of Medical SciencesAbstract
OBJECTIVES. The content of C-Fos protein was tested in rat pinealocytes in the norm and stress and in case of intranasal administration of Epitalon (Ala-Glu-Asp-Gly), which regulated pineal secretion processes, presumably, via protooncogenes.
SETTING. Intact and osmotic-stress-exposed rats were used for the immunohistochemical detection of C-Fos protein. All animals were intranasally administered with Epitalon, the last infusion made in two hours before the biopsy. Simultaneously, light microscopy of the pineal parenchyma was performed in all groups of animals.
RESULTS. A slight but significant C-Fos increase was observed only in stress-exposed pinealocytes of rats after intranasal Epitalon infusions. C-Fos was irregularly distributed throughout pineal cells. In stress, the clusters of 5-10 cells containing C-Fos in their cytoplasm were detected. The dilation of capillaries and pericapillary space induced by osmotic stress was partially reduced by Epitalon intranasal infusions.
CONCLUSIONS. Tetrapeptide Epitalon synthesised on the basis of the amino acid composition of pineal peptide extract Epithalamin modulates pineal secretion only under stress but never in the norm. It prevents osmotic-stress-induced pathologic changes in the structure of the pineal parenchyma. Besides, the physiological activity of Epitalon seems to be mediated by the activation of protooncogenes in pinealocytes.Key words: pinealocytes; Epitalon; C-Fos; oncogenes; osmotic stress.
Introduction
Both the pineal gland and the hypothalamus-hypophysis complex take part in the formation of general adaptation syndrome [1, 2]. Stress causes the pineal gland to increase the secretion of melatonin and peptide substances [2, 11], which are known to reduce the damaging effect of oxygenic, osmotic, psychic and other stresses. The peak of pineal melatonin secretion occurs only at night, while in the daytime pinealocytes show the secretion of other substances, peptides in particular. Yet, the works dedicated to pineal peptides are disproportionally few as compared to publications on melatonin.
The pineal gland belongs to circumventricular organs having no blood-brain barrier. Consequently, this organ is highly sensitive to macromolecular biologically active substances circulating with the cerebral blood flow and, especially, to peptides. The unique location of the olfactory system, its chemical links both to the environment and the central nervous system turn it to a convenient pathway for the non-invasive delivery of substances to the cerebral blood flow and circumventricular organs. This pathway bases on the anatomic connection of the nasal submucosa to the subarachnoid space surrounding olfactory nerves as they penetrate the cribriform plate of the skull and enter the brain [6]. The cribriform region has no significant barrier to cerebrospinal fluid drainage [3, 8]. This is the possible way for metals, dyes, viruses, peptides [14, 15], proteins and narcotics to enter the brain via nasal cavity avoiding the blood-brain barrier [4]. Previously we have shown that intranasally infused epiphyseal peptides reach the pineal gland and specifically regulate its electric activity and pinealocytic ultrastructure. Furthermore, these effects have been observed only in stress, but not in the norm [20]. An important role in intracellular pineal synthesis activation, for example, in stress, belongs by heterodimer AP-1 formed of C-Fos and C-Jun transcription factors [10, 13]. C-Fos protein exists longer than its mRNA. The peak of its content in the cytoplasm is usually observed in two hours after, for instance, a stress impact. Some authors [12] have suggested cytomedins to influence the cells through C-Fos synthesis activation. To prove the hypothesis on the participation of oncogenes in the pineal humoral self-regulation, we have performed an immunohistochemical detection of C-Fos protein in the pinealocytes of stress-exposed rats intranasally administered with Epitalon.
Material and Methods
To detect C-Fos in pinealocytes, we used male Wistar rats – intact ones and those exposed to a 24-hours’ osmotic-stress (n=24). Twelve rats had an unlimited access to water and food: six of them served as the control and the other six were administered with four intranasal Epitalon infusions at 12-hours’ intervals, at the dose of 50µg (0.5 µg per animal). Twelve rats were deprived of water and food for 8 hours: six of them received Epitalon infusions by the same scheme and at the same dose. In two hours after the last infusion, the animals were decapitated (according to FELASA guidelines), their pineal glands were subjected to a standard histological procession and embedded in paraffin. Microtome was used to make 5 micrometer sections for the indirect immunohistochemical detection of C-Fos: the sections were incubated with rabbit C-Fos antibodies (Santa-Cruz, USA) and subsequently with secondary anti-rabbit antibodies conjugated with horseradish peroxidase (Sigma). Peroxidase was revealed with a diaminobenzidine-H2O2 mixture. The stained sections were digitised in a microscope with a 48-bit CCD camera. The presence of C-Fos protein was confirmed by the appearance of the characteristic brown colour.
Simultaneously, the sections were dyed with methylene blue – azure – fuxine. Digitising was done the same way as above. Morphometry was performed with “Ista Video-Test” software (Russia) and in “SigmaScan Professional 5” (SPSS Inc., USA).
Results and Discussion
C-Fos protein belongs to the triggers activating pineal synthetic processes in response to extreme factors, in particular, to stress [10]. In our experiments, the nearly complete absence of C-Fos in the rats’ pineal glands was probably associated with the chronic type of the stress, since according to the published data, the maximum C-Fos content was usually observed in 1-2 hours after a short stress impact [16, 17]. Chronic stress suppressed C-Fos gene activity in the paraventricular hypothalamic nucleus [5] due to the high blood level of glucocorticoids [9, 22]. Besides, the nuclear glucocorticoid receptor and AP-1 transcription factor revealed a regulatory antagonism [21].
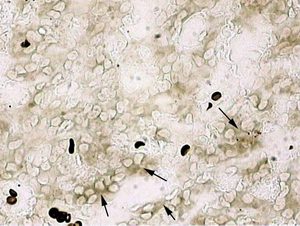
A slight but statistically significant C-Fos increase was observed only in stress-exposed rat pinealocytes after intranasal Epitalon infusions (Figure 1). The obtained results complied with the hypothesis [12] on the possible mechanism of action of cytomedins and their components via C-Fos activation. The fact that Epitalon (a derivative of pineal regulatory peptides) exerted this effect only in stress confirms its participation in the specific mechanisms of pineal self-regulation under extreme conditions.
Only some pinealocytes were found to produce C-Fos. In stress, the clusters of 5-10 C-Fos synthesising cells were registered. Presumably, these clusters represented the very groups of interacting pinealocytes, which we had discovered before in our electrophysiological investigations [18, 19]. Certainly, this assumption would require a more valuable experimental confirmation in the future.
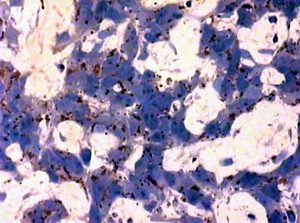
C-Fos detection was accompanied by pineal parenchyma morphometry. The dilation of capillaries and pericapillary space induced by osmotic stress (Figure 2) was partially suppressed with intranasal Epitalon application. This activity of Epitalon resembled its effect upon the gamma-irradiated pineal gland of rats described elsewhere [7].
Thus, synthetic peptide Epitalon (a derivative of pineal regulatory peptides) modulates pineal oncogenes only in stress but not in the norm. It also prevents osmotic-stress-induced pathologic changes in the structure of the pineal parenchyma. Besides, the effect of Epitalon on the pineal gland is probably mediated by the activation of protooncogenes.
References
1. Anisimov VN. Physiological functions of pineal gland (gerontological aspects). Ross Fiziol Zh Im I M Sechenova, 1997; 83(8): 1-13.
2. Arushanyan EB, Baturin VA, Ovanesov KB. Influence of pineal peptides on the dynamics of the circadian and minute motor biorhythms in rats. Neurosci. Behav. Physiol., 1991; 21(2): 145-149.
3. Bradbury MWB, Cser HF. Drainage of cerebral interstitial fluid and of cerebrospinal fluid into lymphatics. In: Experimental biology of the lymphatic Circulation, Johnson M.G. (ed), Elsevier, N.Y; 1985: 356-394.
4. Chen X, et al. Delivery of Nerve Growth Factor to the Brain via the Olfactory Pathway. J. of Alzheimer’s Disease, 1998; 1: 35-44.
5. Chen X, Herbert J. Regional changes in c-fos expression in the basal forebrain and brainstem during adaptation to repeated stress: correlations with cardiovascular, hypothermic and endocrine responses. Neuroscience, 1995; 64: 675-685.
6. Frey II WH, et al. Delivery of 115I-NGF to brain via Olfactory Route. Drug Delivery, 1997; 4: 87-92.
7. Khavinson VK, et al. Reparative effect of Epitalon on pineal gland ultrastructure in gamma-irradiated rats. Bull Exp Biol Med, 2001; 131(1): 85-101.
8. Kida S, Pantazis A, Weller RO. CSF drains directly from the subarachnoid space into nasal lympatics in the rat. Anatomy, histology and immunological significance. Neuropathology and Applied Neurobiology, 1993; 19: 480-488.
9. Kovaks KJ, Sawchenko PE. Glucocorticoid negative feedback is exerted selectively on vasopressin gene transcription in parvocellular neurosecretory neurons. Soc. Neurosci. Abstr., 1997; 27: #797.8.
10. Kovaks KJ. c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem. Int., 1998; 33: 278-297.
11. Kovalenko RI. Sibarov DA, Pavlenko IN, Lukianova EL. The structure of rat pinealocytes in stress and after unilateral intranasal administrations of oxytocin. Ross Fiziol Zh Im I M Sechenova, 1997; 83(8): 78-93.
12. Kuznik BI, Morozov VG. Cytomedins. St. Petersburg, Nauka, 1998.
13. Palceva MA, Ivanova AA, Intercellular interactions, Moscow, Medicine, 1995.
14. Pietriwski R, Thiemann A, Kern W, Fehm H, Born J. A nose-brain pathway for psychotropic peptides: evidence from a brain evoked potential study with cholecystokinine. Psychoneuroendocrinology, 1996b; 21: 559-572.
15. Pietriwsky R, Struben C, Molle M, Fehm HL. Brain potential changes after intranasal vs. intravenous administration of vasopressin: evidence for a direct nose-brain effect in humans. Biological Psychiatry, 1996a; 39: 332-340.
16. Sasson-Corsi P, Lamph WW, Verma I.M. Cold Spring Harbor Symposia on Quantitative Biology, Cold Spring Harbor, 1989; 53(2): 749-757.
17. Shang SL, Squinto SP, Harlan RE. Biochemical and Biophysical Research Communications, 1988; 157: 698-704.
18. Sibarov DA, Kovalenko RI, Nozdrachev AD. Pinealocyte functioning in stress during daytime in rats. Ross Fiziol Zh Im I M Sechenova, 2000; 86(8):1049-56.
19. Sibarov DA, Kovalenko RI, Anisimov VN, Nozdrachev AD. Daytime pineal gland activation in rats with colon tumors induced by 1,2-dimethylhydrazine. Neuroendocr.Lett., 2000; 21: 307-312.
20. Sibarov DA, Kovalenko RI. Daytime stress-induced pineal gland structural changes. The 28th Gottingen Neurobiology Conference (June 7-10), Gottingen, Germany, 2001; # 1022.
21. Unlap T, Jope PS. Dexamethazone attenuates kainate-induced AP-1 activation in rat brain. Molecular Brain Res., 1994; 24: 275-282.
22. Wan W et al. Differential induction of c-Fos immunoreactivity in hypothalamus and brain stem nuclei following central and peripheral administration of endotoxin. Brain Res. Bull., 1993; 32: 581-587.
Figure LegendsFigure 1. Immunohistochemical detection of C-Fos protein
AB
A – without Epitalon infusion
B – with intranasal Epitalon infusion (cell clusters producing C-Fos protein)
Figure 2. Pineal parenchyma after a 48-hours’ water and food deprivation
A
B
A – without Epitalon infusion
B – after intranasal Epitalon infusion