This page accompanies a discussion of thimerosal under BITN - Miscellaneous; section on vaccines.
So, what is thimerosal? Figure 1 shows its chemical structure.
|
Figure 1.
Thimerosal. |
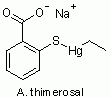
|
Thimerosal is a salicylate (related to salicylic acid) -- perhaps a familiar group of compounds. Figure 2 shows four other members of this family.
|
Figure 2.
Some other salicylates. Note that "C" is salicylic acid itself. |
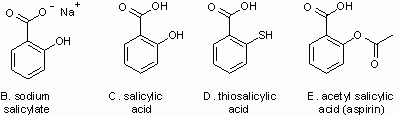
|
Compare structures B and C in Fig 2. The only difference is that one shows the acid, with -OH at the top, and the other shows the sodium salt, with O- Na+. These are readily interconverted, just by changing the pH -- the acidity (Intro Chem book Ch 18). At neutral pH, as in your bloodstream, the salt form is the major form, and that is why thimerosal is usually shown in the salt form. However, it is easier to write the acid form, so we often do -- with the understanding that the user can adjust for pH as needed. I have drawn the other structures here in the acid form.
Now compare structures C and D in Fig 2. The only difference is -OH vs -SH on the side of the ring. The -SH makes this a "thio" structure (thio standing for sulfur). We see that thimerosal itself (A) is this thiosalicylate with -Hg-CH2-CH3 attached to the S. That group is an "ethyl mercury" group. In fact, thimerosal is broken down in the body to ethyl mercury + thiosalicylic acid.
In the name thimerosal, the "thi" refers to the sulfur, the "mer" refers to the mercury, and the "sal" refers to the salicylate.
Structure E in Fig 2 is a common salicylate.