Atomic Radius vs Atomic Number
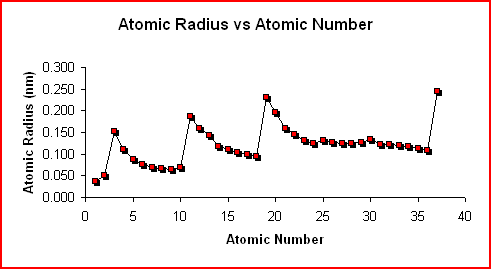
The graph of Atomic Radius vs Atomic Number shows the trend in atomic radius as one proceeds through the first 37 elements in the periodic table.
Within the same period or series, the atomic radius decreases as you move from the alkali metal to the noble gas. This trend results from an increase in the nuclear charge and an increase in the number of electrons in the same principal energy level (n). Increasing the quantity of charge attracts the electrons closer to the nucleus.
Within the same group or family, the atomic radius increases as you go down the group. Although there is an increase in nuclear charge, adding another principal energy level is the predominant factor. The addition of another principal energy level results in the valence electrons being further from the nucleus.
 |