Boyle's Law
Boyle's Law states the relationship between the volume and pressure of a gas as the temperature and the number of moles remain constant. It states that the volume and pressure are inversely proportional.
If the volume is increased on a gas the pressure will decrease and as the volume is decreased the pressure will increase. The first graph shows the inverse relationship between volume and pressure. Mathematically stated, Boyle's Law says that PV = k, where k is a constant for a given volume of gas at constant temperature.
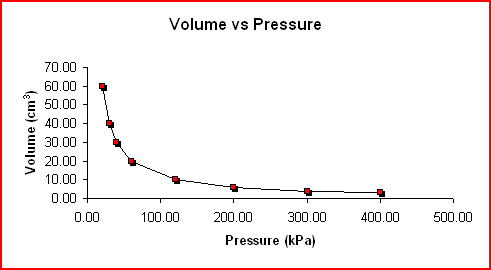 |
The graph below shows the linear relationship when V is plotted versus 1/P.
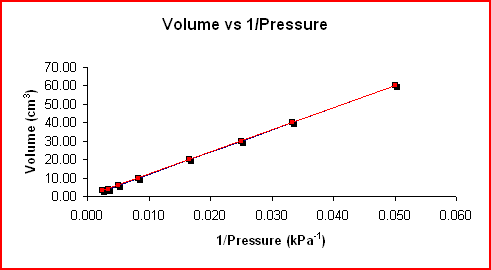 |