Charles's Law
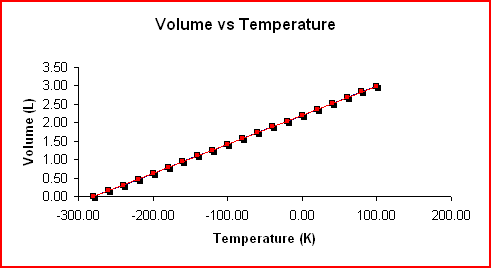
Charles's Law states the relationship between the volume and temperature of a gas as the pressure and the number of moles remain constant. It states that the volume and the Kelvin temperature are directly proportional.
If the volume is doubled the Kelvin temperature will double and if the volume is halved the Kelvin temperature will be halved. Mathematically stated, Charles's Law says that V/T = k, where k is a constant for a given volume of gas at constant pressure.
 |