
1) 1.0 g of each of the following compounds is mixed with 100. mL of water. Which will form solutions that will conduct electricity?
(a) CuS
(b) C2H5OH
(c) MgCl2
(d) Na2C2O4
(e) SO2
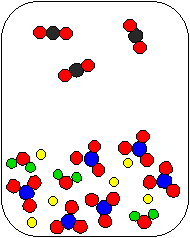
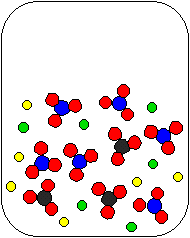
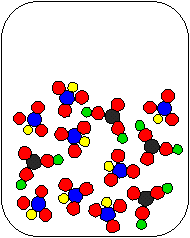
2) Li2CO3(aq) and HNO3(aq) are combined in a beaker. Which of the following diagrams is the most appropriate representation of the contents of the beaker after any reaction occurs? (Water molecules from the aqueous solutions are not shown.)
(a) |
(b) |
(c) |
 |
3) 1.0 g of each of the following compounds is mixed with 100. mL of water. Which will form solutions that will conduct electricity?
(a) CH3OH
(b) MgO
(c) AgCl
(d) KCN
(e) CO2
4) MgSO3 and HClO4(aq) are combined in a beaker. Which of the following diagrams is the most appropriate representation of the contents of the beaker after any reaction occurs? (Water molecules from the aqueous solutions are not shown.)
(a) (b) (c)
5) 1.0 g of each of the following compounds is mixed with 100. mL of water. Which will form solutions that will conduct electricity?
(a) Cu(OH)2
(b) C3H8
(c) NaNO3
(d) Ca(C2H3O2)2
(e) PCl5
6) NiSO4(aq) and Li2CO3(aq) are combined in a beaker. Which of the following diagrams is the most appropriate representation of the contents of the beaker after any reaction occurs? (Water molecules from the aqueous solutions are not shown.)
(a) (b) (c)
7) 1.0 g of each of the following compounds is mixed with 100. mL of water. Which will form solutions that will conduct electricity?
(a) CaC2O4
(b) C2H6O
(c) Li2CO3
(d) BaSO4
(e) PF3
8) Cu(NO3)2(aq) and K2S(aq) are combined in a beaker. Which of the following diagrams is the most appropriate representation of the contents of the beaker after any reaction occurs? (Water molecules from the aqueous solutions are not shown.)
(a)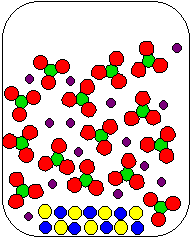 |
(b)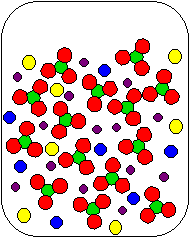 |
(c)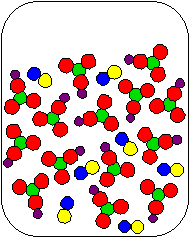 |
 |
9) 1.0 g of each of the following compounds is mixed with 100. mL of water. Which will form solutions that will conduct electricity?
(a) PbS
(b) C3H7OH
(c) Mg(ClO4)2
(d) Al(OH)3
(e) NOBr
10) Ca(OH)2(aq) and Na2SO4(aq) are combined in a beaker. Which of the following diagrams is the most appropriate representation of the contents of the beaker after any reaction occurs? (Water molecules from the aqueous solutions are not shown.)
(a)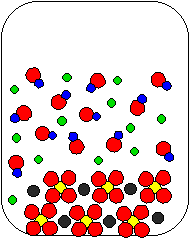 |
(b)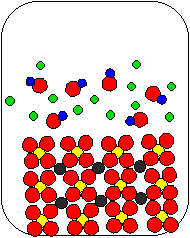 |
(c)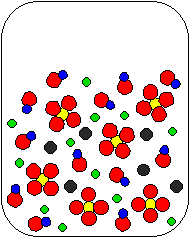 |
 |