The Determination of Keq for FeSCN2+
![]()
A chemical system is said to be in equilibrium when there are no measurable changes occurring. An example of chemical equilibrium is shown below:
aA + bB <---> cC + dD
When reactants A and B are first mixed, the system is not in equilibrium. As the reaction proceeds, the decrease in the concentration of the reactants can be measured as well as an increase in the concentration of the products. Because all reactions are reversible to some degree, as soon as products C and D are formed, they react in reverse to form products A and B again as shown below:
cC + dD <---> aA + bB
The rate of a reaction generally varies with the concentration of reactants, i.e. decreasing when the reactant concentrations decrease, and increasing when the reactant concentrations increase.
Eventually, a condition of static concentrations or an equilibrium state is reached. When the position of the equilibrium is far to the reactant side, it is said that there is no observable reaction. When the position of the equilibrium is far to the product side, it is said that the reaction goes nearly to completion. For a system to be in equilibrium means that the concentrations of all chemical species do not change with time and the temperature, volume, and pressure remain constant. However, on a molecular scale, equilibrium is not static but a dynamic condition, where chemical changes are still occurring but are not detectable.
Both the forward and reverse reactions continue to occur at equilibrium. Because their rates are equal, there is no net change in reactant concentrations. Once equilibrium is reached, it is maintained only if all relevant factors remain the same. A change in reactant concentrations, temperature, pressure, or volume will disturb the equilibrium causing the system to undergo additional net changes to establish a new equilibrium.
In Part A of this lab, a series of reference solutions are prepared by reacting large amounts of Fe3+ (iron(III) ions) with known amounts of SCN- (thiocyanate) ions. The test solutions are prepared by mixing constant amounts of Fe3+ ions with different amounts of SCN- ions. The test solutions contain unknown concentrations of FeSCN2+ ions at equilibrium. Both solutions represent the reversible reaction shown below:
Fe3+(aq) + SCN-(aq) <---> FeSCN2+(aq)
Using colorimetry, the absorbance values of the reference solutions are determined as shown in the data table below.
Sample |
[FeSCN2+] (M) |
Absorbance |
Ref Solution 1 |
4.0 x 10-5 |
0.170 |
Ref Solution 2 |
6.0 x 10-5 |
0.255 |
Ref Solution 3 |
8.0 x 10-5 |
0.362 |
Ref Solution 4 |
1.0 x 10-4 |
0.430 |
Ref Solution 5 |
1.2 x 10-4 |
0.512 |
The sample data above was used to produce the calibration curve for the absorbance of FeSCN2+. A calibration curve is made by plotting Absorbance (at a particular wavelength) vs [FeSCN2+] of the reference solutions.
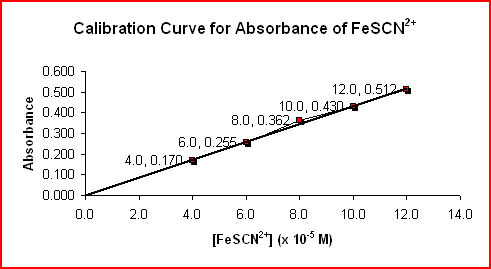 |
By using the absorbance readings of the test solutions, the calibration curve is used to determine the unknown concentrations of FeSCN2+.
©2004, Flinn Scientific, Inc. All Rights Reserved. Reproduced for one-time use with permission from Flinn Scientific, Inc., Batavia, IL, USA. No part of this material may be reproduced or transmitted in any form or by any means, electronic or mechanical, including, but not limited to photocopy, recording, or any information storage and retrieval system, without permission in writing from Flinn Scientific, Inc.; and
That the Licensee agrees to indemnify and hold Flinn Scientific, Inc. harmless from any and all liability, loss, damages, costs of expense which Flinn Scientific, Inc. may hereafter incur, suffer or be required to pay by reason of such publication by the Licensee; and
To compensate Flinn Scientific, Inc. $0.00 for the permission to reproduce this material.