Thermodynamics - Enthalpy Of Reaction And Hess's Law
![]()
This lab demonstrates the principle of Hess's law. Hess's law states that the change in enthalpy is the same whether a reaction takes place in one step or in a series of steps. This implies that enthalpy is a state function and is independent of the specific path or the series of steps carried out in a chemical reaction.
The following reactions will take place in this lab:
(1) NaOH(aq) + HCl(aq) ---> NaCl(aq) + H2O(l)(2) NH4Cl(aq) + NaOH(aq) ---> NH3(aq) + NaCl(aq) + H2O(l)
(3) NH3(aq) + HCl(aq) ---> NH4Cl(aq)
According to Hess's law, the enthalpy change, ΔHrxn, for the 3rd reaction equals the sum of the ΔHrxn for reactions 1 and 2, after the 2nd reaction is reversed..
Because each of the reactions are measured for temperature change by calorimetry a Temperature vs Time graph will be drawn to obtain an accurate Tmix, the initial temperature of the mixtures.
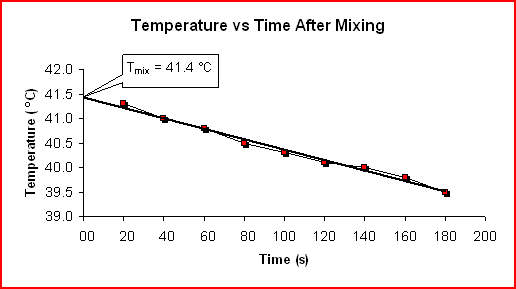
Determining The Heat Capacity Of The Calorimeter:
Time Temp (s) (C) 20 41.3 40 41.0 60 40.8 80 40.5 100 40.3 120 40.1 140 40.0 160 39.8 180 39.5
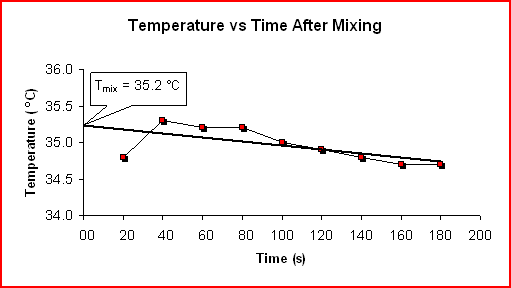
Determining ΔH for NaOH(aq) + HCl(aq) ---> NaCl(aq) + H2O(l):
Time Temp (s) (C) 20 34.8 40 35.3 60 35.2 80 35.2 100 35.0 120 34.9 140 34.8 160 34.7 180 34.7
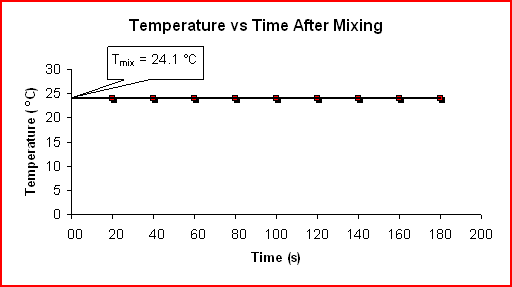
Determining ΔH for NH4Cl(aq) + NaOH(aq) ---> NH3(aq) + NaCl(aq) + H2O(l):
Time Temp (s) (C) 20 24.1 40 24.1 60 24.1 80 24.1 100 24.1 120 24.1 140 24.1 160 24.1 180 24.1
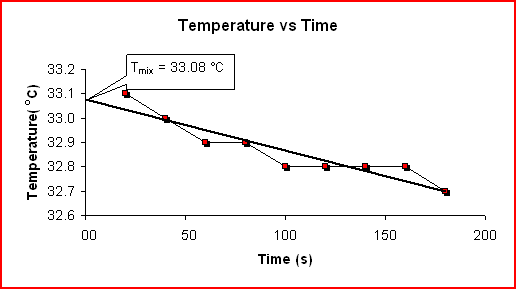
Determining ΔH for NH3(aq) + HCl(aq) ---> NH4Cl(aq):
Time Temp (s) (C) 20 33.1 40 33.0 60 32.9 80 32.9 100 32.8 120 32.8 140 32.8 160 32.8 180 32.7
©2004, Flinn Scientific, Inc. All Rights Reserved. Reproduced for one-time use with permission from Flinn Scientific, Inc., Batavia, IL, USA. No part of this material may be reproduced or transmitted in any form or by any means, electronic or mechanical, including, but not limited to photocopy, recording, or any information storage and retrieval system, without permission in writing from Flinn Scientific, Inc.; and
That the Licensee agrees to indemnify and hold Flinn Scientific, Inc. harmless from any and all liability, loss, damages, costs of expense which Flinn Scientific, Inc. may hereafter incur, suffer or be required to pay by reason of such publication by the Licensee; and
To compensate Flinn Scientific, Inc. $0.00 for the permission to reproduce this material.