Ionization Energy vs Atomic Number
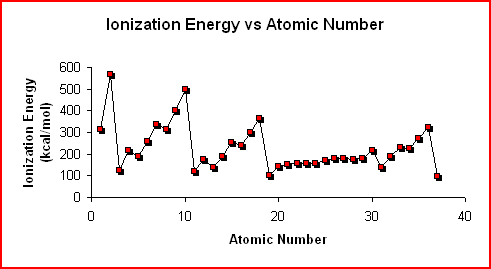
The graph of Ionization Energy vs Atomic Number shows the trend in the first ionization energy as one proceeds through the first 37 elements in the periodic table.
Within the same period or series, the first ionization energy generally increases as you move from the alkali metal to the noble gas. This trend results from the same two factors that determine atomic radius. As the nuclear charge increases and the atomic radius decreases the increased attraction makes it more difficult to remove an electron.
Within the same group or family, the first ionization energy decreases as you go down the group. Although there is an increase in nuclear charge, adding another principal energy level is the predominant factor. The addition of another principal energy level results in the electron being further from the nucleus and the decreased attraction makes it easier to remove an electron.
 |