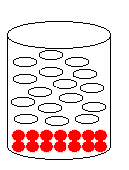
1) Which of the following solutions corresponds
to the molecular diagram shown to the right? 
(a) water and silver chloride
(b) water and octane
(c) water and lead(II) sulfate
(d) water and ethanol
| 
| 
|
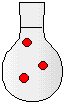
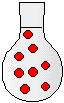
500 mL | 500 mL | 500 mL |

| 
| 
|
250 mL | 250 mL | 250 mL |
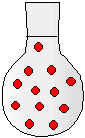
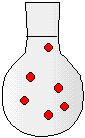
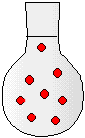
2) The drawings to the right represent flasks of aqueous solutions. Each red dot represents a dissolved solute particle. If you place solution D in a 500-mL flask and dilute to a volume of 500. mL, which flask best represents the new solution?
(a) Solution A
(b) Solution B
(c) Solution C
(d) Solution D
(e) Solution E
(f) Solution F
3) Which of the following solutions corresponds
to the molecular diagram shown to the right? 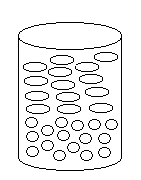
(a) acetic acid and hexane
(b) ethanol and water
(c) water and octane
(d) water and methanol
4) Which of the following solutions corresponds
to the molecular diagram shown to the right? 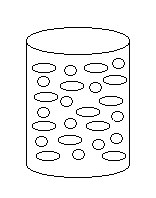
(a) acetic acid and hexane
(b) ethanol and water
(c) water and octane
(d) water and methanol

| 
| 
|
500 mL | 500 mL | 500 mL |

| 
| 
|
250 mL | 250 mL | 250 mL |
5) The drawings to the right represent flasks of aqueous solutions. Each red dot represents a dissolved solute particle. Which of the following solutions is most concentrated?
(a) Solution A
(b) Solution B
(c) Solution C
(d) Solution D
(e) Solution E
(f) Solution F
6) Which of the following solutions corresponds
to the molecular diagram shown to the right? 
(a) formic acid and water
(b) ethanol and methanol
(c) water and bromine
(d) water and hexane