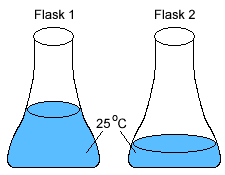

1) If 200. J of thermal energy was added to both flasks, which of the following would happen?
(a) The final temperature of flask 1 would be greater than that of flask 2.
(b) The final temperature of flask 1 would be lower than that of flask 2.
(c) The final temperatures of flask 1 and 2 would be the same.
(d) The water in both flasks would boil away.
2) Two identical flasks with different volumes of water
at the same temperature. 
(a) Flask 1 has more thermal energy than flask 2.
(b) Flask 2 has more thermal energy than flask 1.
(c) Flask 1 and flask 2 have the same amount of thermal energy.
3) If 200. J of thermal energy was removed from both flasks,
which of the following would happen?
(a) The final temperature of flask 1 would be greater than that of flask 2.
(b) The final temperature of flask 1 would be lower than that of flask 2.
(c) The final temperatures of flask 1 and 2 would be the same.
(d) The water in both flasks would freeze.
4) Two identical flasks with different volumes of
water at the same temperature. 
(a) Flask 1 has less thermal energy than flask 2.
(b) Flask 2 has less thermal energy than flask 1.
(c) Flask 1 and flask 2 have the same amount of thermal energy.
5) If 25.0 mL of water at 25°C were added to both
flasks, which of the following would happen? 
(a) The final temperature of flask 1 would be greater than that of flask 2.
(b) The final temperature of flask 1 would be lower than that of flask 2.
(c) The final temperature of flask 1 and flask 2 would be the same.
6) If 25.0 mL of water at 25°C were removed from both
flasks, which of the following would happen? 
(a) The final temperature of flask 1 would be greater than that of flask 2.
(b) The final temperature of flask 1 would be lower than that of flask 2.
(c) The final temperature of flask 1 and flask 2 would be the same.
7) If 25.0 mL of water at 80°C were added to
both flasks, which of the following would happen? 
(a) The final temperature of flask 1 would be greater than that of flask 2.
(b) The final temperature of flask 1 would be lower than that of flask 2.
(c) The final temperature of flask 1 and flask 2 would be the same.
8) If 25.0 mL of water at 4°C were added to
both flasks, which of the following would happen?
(a) The final temperature of flask 1 would be greater than that of flask 2.
(b) The final temperature of flask 1 would be lower than that of flask 2.
(c) The final temperature of flask 1 and flask 2 would be the same.
.