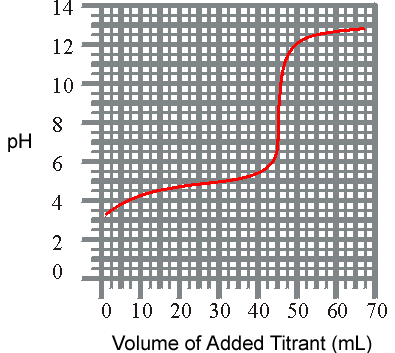
1) The titration of a weak acid with a strong base is shown below. What is the approxiamate Ka for the acid?
pH vs Volume

(a) 1.8 x 10-5
(b) 6.3 x 10-4
(c) 3.6 x 10-6
(d) 3.2 x 10-9
2) The titration of a weak base with a strong acid is shown below. What is the approxiamate Kb for the base?
pH vs Volume
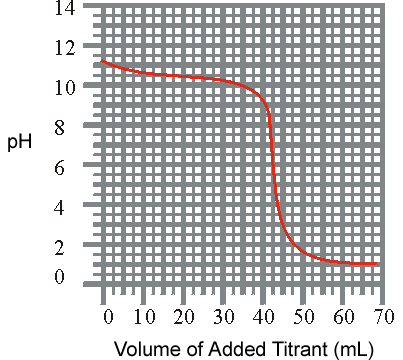
(a) 3.1 x 10-4
(b) 3.2 x 10-11
(c) 1.0 x 10-8
(d) 1.0 x 10-6
3) The titration of a weak acid with a strong base is shown below. What is the approxiamate Ka for the acid?
pH vs Volume
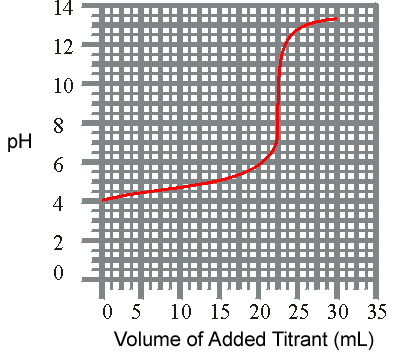
(a) 8.8 x 10-2
(b) 1.0 x 10-4
(c) 1.6 x 10-9
(d) 1.6 x 10-5
4) The titration of a weak base with a strong acid is shown below. What is the approxiamate Kb for the base?
pH vs Volume
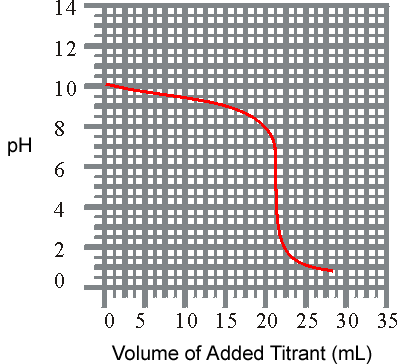
(a) 7.9 x 10-11
(b) 2.5 x 10-5
(c) 1.3 x 10-4
(d) 4.0 x 10-10