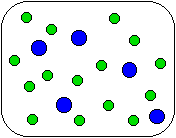
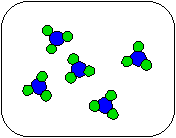
1) Shown below is a compound reacting to form products. Which equation best describes the stoichiometry of the reaction?
 |  |  |
(a) A + 3B
AB3
(b) A + B
AB3
(c) 5A + 5B
5AB
(d) 5A + 15B
5AB3
2) According to the law of conservation of mass, which of the following diagrams represent possible products for the following reaction?
A2 + 2B2 ![]()
(a) 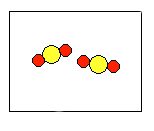 |
(b) 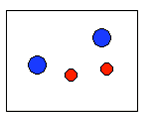 |
(c) 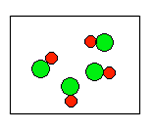 |
(d) 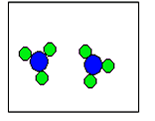 |
3) Shown below is a compound reacting to form products. Which equation best describes the stoichiometry of the reaction?
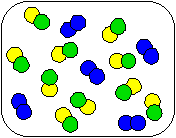 |  | 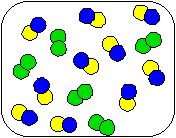 |
(a) AB + 2C
AC + 2B
(b) 2AB + C2
2AC + B2
(c) AB + C2
AC + B2
(d) 10AB + 5C2
10AC + 5B2
4) According to the law of conservation of mass, which of the following diagrams represent possible products for the following reaction?
3A2 + 2B2 ![]()
(a) 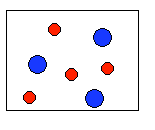 |
(b) 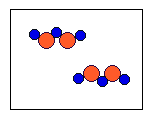 |
(c)  |
(d) 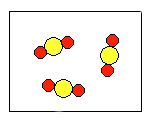 |
5) According to the law of conservation of mass, which of the following diagrams represent possible products for the following reaction?
2A3 + 3B ![]()
(a)  |
(b)  |
(c)  |
(d)  |
6) According to the law of conservation of mass, which of the following diagrams represent possible products for the following reaction?
A3 + 6B ![]()
(a) 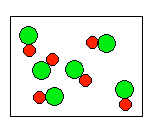 |
(b) 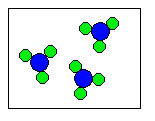 |
(c) 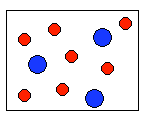 |
(d)  |
7) According to the law of conservation of mass, which of the following diagrams represent possible products for the following reaction?
A2 + 4B2![]()
(a)  |
(b)  |
(c) 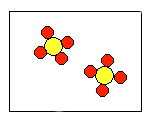 |
(d)  |