ABSTRACT:
We have designed new vectors for the construction of recombinant adenoviruses containing the early region 1A (E1A) gene under the transcriptional control of heterologous promoters. The normal E1A regulatory elements have been replaced by a convenient set of unique restriction enzyme sites, allowing for introduction of gene regulatory cassettes with relative ease. Subsequent rescue generates recombinant conditionally-replicating adenovirus in which the transcription of E1A is under alternative control. This allows cell type-specific expression of E1A, restricting efficient virus replication to chosen target cells.
INTRODUCTION:
Recent work has highlighted the use of replication-competent viruses as a potential treatment for cancer. This approach capitalizes on the ability of the virus to replicate itself, producing a multitude of infectious progeny, with the concomitant death of the targeted host cancer cell. This produces an infection capable of killing cells in the primary tumor that were not originally infected and potentially even cells that have metastasized to distant sites.
Adenoviral early region 1A (E1A) gene products function to activate transcription of other viral gene products and to create conditions within the host cell favorable to efficient production of progeny. These functions of E1A are indispensable for normal lytic infection, and viruses that fail to express functional E1A products are considered replication-incompetent. For this reason, viruses with altered patterns of E1A expression or altered E1A function have been studied as candidate oncolytic replication-competent adenoviruses (ORCA).
We report here the construction of new vectors for conveniently generating recombinant adenoviruses with alternative transcriptional elements regulating expression of E1A. Using these vectors, we have constructed recombinant viruses with the well-characterized human Cytomegalovirus Immediate Early (CMV IE) and Mouse Mammary Tumor Virus (MMTV) transcriptional elements regulating E1A expression. We show that E1A expression after infection with these viruses is indeed regulated as predicted, demonstrating the utility of these vectors.
VECTORS

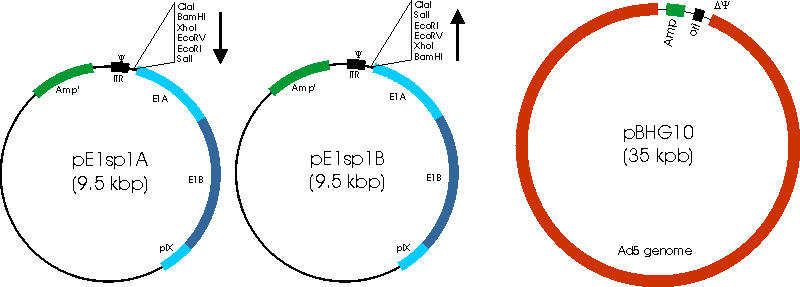
* The pE1sp1X vectors contain the adenoviral packaging signal (Ψ), the E1A, E1B and pIX genes, as well as a convenient set of restriction sites in place of bp340-556 of the Ad5 genome
* The pBHG10 vector contains full adenoviral genome lacking only the packaging signal (ΔΨ)
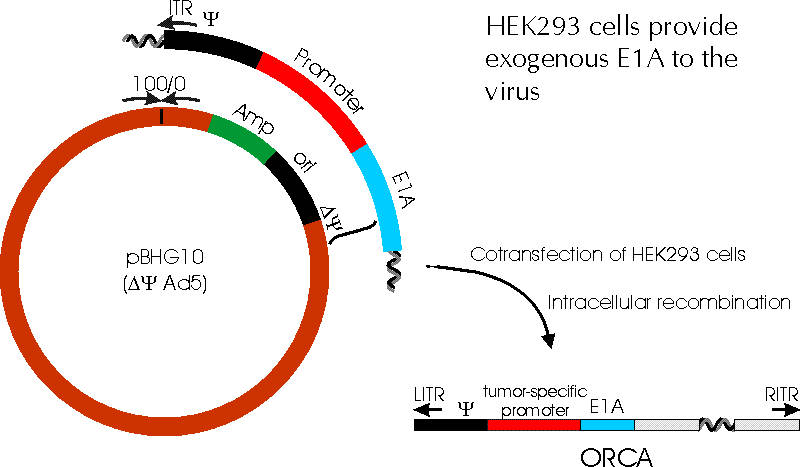
* Recombination between pBHG10 and pE1sp1X must occur to reconstitute the complete viral genome
RECOMBINATION STRATEGY
(as described in Bett et al., PNAS Vol 91, 1994)
PROOF OF CONCEPT: TEST REGULATORY REGIONS
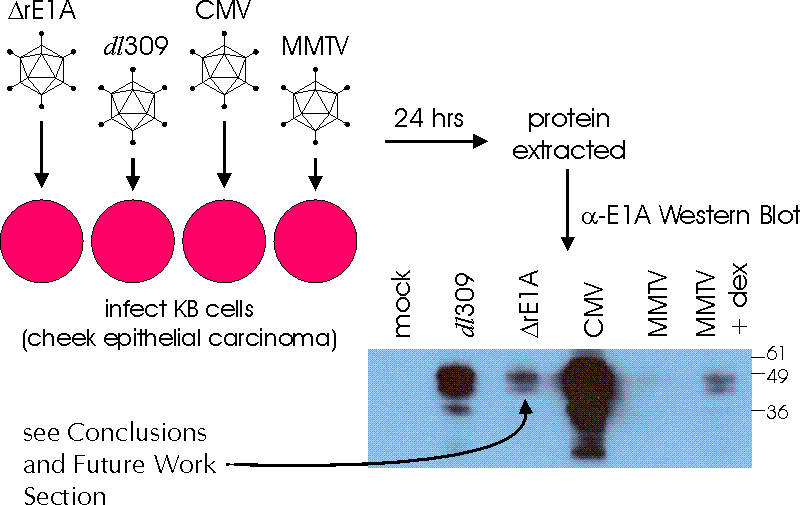
*Cytomegalovirus Immediate Early (CMV IE) - strong constitutive promoter
*Mouse Mammary Tumor Virus (MMTV) Long Terminal Repeat (LTR) - glucocorticoid-inducible promoter
*ΔrE1A - virus lacking normal E1A transcriptional control regions
*dl309 - virus with wild-type E1A transcriptional control regions
ANALYSIS OF E1A PROTEIN LEVELS IN KB CELLS INFECTED WITH ORCA VIRUSES
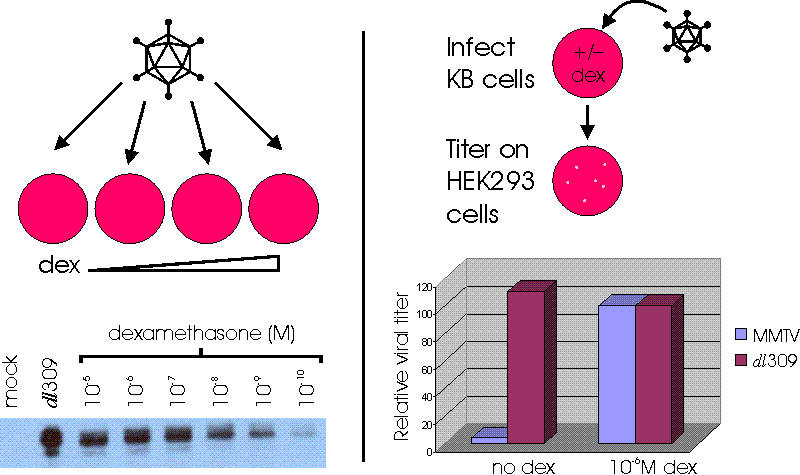
REGULATION OF THE MMTV ORCA BY THE GLUCOCORTICOID DEXAMETHASONE
CONCLUSIONS:
* A new set of vectors has been constructed for convenient generation of replication-competent viruses with altered E1A expression
* Proof-of-concept experiments indicate that expression of E1A in rescued viruses is regulated by heterologous promoters in the expected manner
* Altered expression of E1A results in controllable production of viral progeny
* * transcription initiation sites within the packaging signal result in low levels of E1A expression in the absence of the normal E1A regulatory elements or a heterologous promoter
FUTURE WORK:
* alternative transcription initiation sites within the packaging signal can be silenced by insertion of polyadenylation sequences upstream of the MCS (Massuda et al., PNAS Vol 94, 1997)
* construction of ORCAs with tissue- and “tumor-specific” promoters and testing in appropriate cell lines [e.g. Prostate Specific Antigen (PSA) and kallikrein (prostate), tyrosinase (melanoma), MUC1 (breast cancer), L-plastin (ovarian cancer)]
* evaluation of clinical potential in mice with grafted tumors
ACKNOWLEGEMENT:
This research was supported by the Internal Research Fund of the London Health Sciences Centre.