MINERAL PROCESSING SITEby Eren Caner ORHAN |
This site is visited times since 15 October 1997
The ores with sulfide minerals often consists of metal sulfide minerals and gangue. However, rarely, only the separation of a sulfide from the gangue can be requested. This is the simplest application of flotation which is called selective flotation. Besides, the separation of all of the sulfides from the gangue is called bulk flotation. Sulfide minerals such as those of copper (CuFeS2), lead (PbS), and zinc (ZnS) are commonly found in the same ore. Xanthates (dithiocarbonates) and dithiophosphates are used as collectors in sulfide mineral flotation; frothers include pine oil and synthetic commercial compounds such as Dowfroth 250. Some qualified generalizations apply to sulfide mineral flotation:
In the flotation of an ore consisting of chalcopyrite (CuFeS2), galena (PbS), sphalerite (ZnS), pyrite (FeS2), and gangue minerals following procedures are in use:
Selectivity between sulfide minerals is possible when one can adsorb collector, and the others cannot.
|
|
Figure 1 shows contact angle data for three sulfide minerals. In this figure, flotation is possible to the left of each curve, but not to the right. Clearly, as the pH is increased, depression occurs. For pyrite when the pH is raised above about 6,the rest potential no longer exceeds the reversible potential for the oxidation of the collector ion; and also, the Fe-collector compound is not as stable as competing Fe-hydroxyl species. With galena, the Pb-collector compound prevails until the pH reaches about 8; above this value, it is Pb hydroxyl species that predominate. From inspection of Fig. 1, it is clear that selective flotation is possible based on the depressant action of the hydroxyl ion alone. However, although these results give an indication of possible flotation operating conditions, it must be kept in mind that they were obtained by contact angle tests on clean specimens under ideal conditions. In many sulfide systems, pH regulation alone is insufficient for acceptable selectivity.
The principle of using competing ions, however, can be used to improve selectivity. In Fig. 2, the depressant effect of cyanide ions is shown by contact angle curves, flotation being possible to the left of each curve. The mechanisms of depression are more complex than was thought in the past.
In the following table, the data obtained from rougher flotation cells of copper, is given as a relation of time with cumulative percent recovery for different average particle sizes. Evaluate the data with graphs and make a comment.
Time |
% Recovery |
|||
| 53 (mm) | 180 (mm) |
200 (mm) |
300 (mm) |
|
0.25 |
15 |
48 |
28 |
5 |
0.5 |
25 |
72 |
50 |
9 |
1.0 |
34 |
84 |
62 |
12 |
1.5 |
40 |
90 |
70 |
14 |
2.5 |
45 |
92 |
76 |
15 |
3.5 |
50 |
94 |
78 |
16 |
5.0 |
58 |
95 |
82 |
17 |
7.0 |
62 |
96 |
84 |
18 |
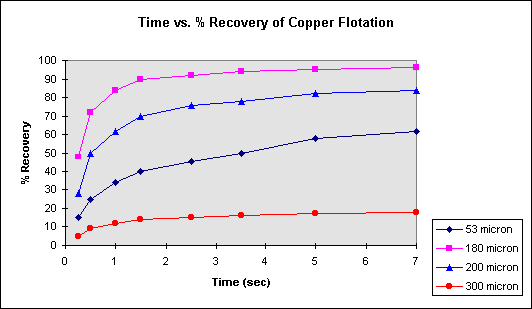
According to the graphical data, it is observed that the recovery of copper is highest at 180 µ m size. Besides, the low recovery at 300 µ m is a result of the minerals not adsorbing to the air bubbles as they are too large and heavy to float. The reason of the low recovery at smaller sizes like 53 µ m is that they are too small and the mineral has a slimy character. These small particles can come together and form larger particles which would be larger than the bubbles can float.
Eren Caner
ORHAN
Hacettepe University
Ankara,Turkey