MINERAL PROCESSING SITEby Eren Caner ORHAN |
This site is visited times since 15 October
1997
Colloidal particles are usually contaminated by electrostatic adsorption of ions to the surface. This primary adsorption layer gives rise to a substantial surface charge (ie. electric potential at the surface). This surface charge does two important things:
Thus, an "ion cloud" extends into solution around a charged surface effectively "balancing" the surface charge over some distance away from particles. The thickness of this electric double layer (ion cloud) around colloidal particles determines how close two particles can get to each other before they start experiencing repulsive forces. The size of this "ion cloud" depends on several factors:
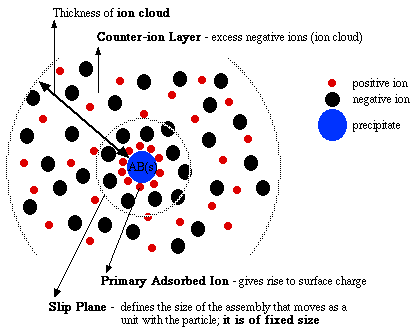
Figure 1. The "Ion Cloud" and the "Slip Plane".
The volume defined by the entire ion cloud surrounding a particle and that defined by the slip plane for a particle are not the same things.
Counter-Ion Layer (Ion Cloud) Thickness: The thickness of the solution layer around the particle that is required so as to contain enough counter-ions to "balance" the surface charge.
Slip Plane Thickness: the thickness of the solvent/ion film which moves with the particle.
The electrical double layer model was first introduced by Stern (1924) by combining the Helmholtz and the Gouy-Chapman models. According to Stern about one molecul distance the presence of a layer, Stern layer, is considered. The oppositely charged ions in this layer decreases the surface potential linearly. Besides, there is another layer which consists of the other scattered ions. Here, the potential decrease is not linear, but approaches to zero as in the Figure 2, that means the ion concentration decreases with the increase of the distance and reaches to the normal concentration of the solution.
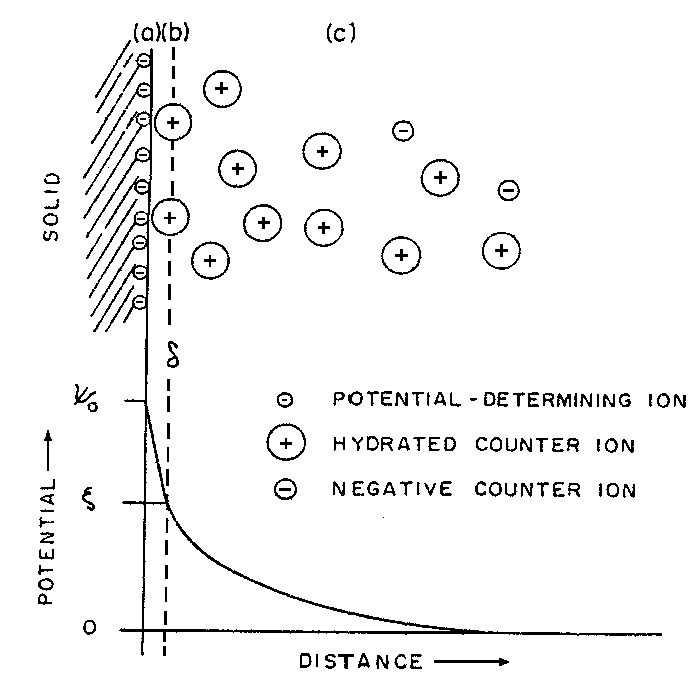 |
Figure 2.
Schematic represantation of the double layer and potential
drop across the double layer;
|
The electrical charge on the solid surface (s S) is the electrical charge in the Stern layer.
The effect of electrolyte concentration on the double layer thickness and thus its effect on colloidal ppt coagulation can be best understood in terms of the zeta potential.
Zeta potential (x ) is the electric potential that exists at the "slip plane" - the interface between the hydrated particle and the bulk solution. It is the measurable potential of a solid surface and also called electrokinetic potential. According to the electrostatic principles zeta potential is calculated by the equation,
x = 4p s d / D
d : thickness of the electrical
double layer
s
: the electrical charge in the Stern layer
D : dielectrical constant.
There are four basic types of electrokinetics effects. Theoretically, each can be utilized to evaluate the zeta potential for a given set of conditions.
The electrical double layer concept is useful in the explanation of the electrostatic behaviours of reagents like collectors, activators, depressants on the mineral surface. In addition to this the zeta potential which is derived from the electrical double layer concept is examined in the pH selection of the flotation process. For instance, in the previous hematite-quartz example, we decided in which pH range the flotation shoul be done and what type of a collector to use.
The relation between the value of the zeta potential and flocculation or dispersion is not exactly determined yet. However, it is experimentally determined that at low zeta potential values flocculation, and at high values of zeta potential dispersion occurs.
Eren Caner
ORHAN
Hacettepe University
Ankara, Turkey