1
Intro
The human species is dependent upon electricity for
the function of our technological society.
We use it to light our world, heat our homes, and to transfer
information, among other things. It is
expected that by 2020, our energy consumption will have increased by 80%, as
the population increases and more nations become technologically
developed. If making technologies more
efficient is concentrated upon, this could possible be lowered to only 40%. Unfortunately, our resources can not sustain
even this amount of energy use forever.
2
Problem
We currently use fossil fuels – coal, oil,
and natural gas -- for most of our energy.
Fossil fuels are created over millions of years from dead organic
matter, and are therefore not renewable energy sources. At the current rate of consumption, we could
find ourselves with a shortage of fossil fuels during this new century. The combustion of these fuels also releases
large amounts of carbon dioxide into the atmosphere, contributing to the greenhouse
effect.
Hydroelectric power is a common alternative
to fossil fuels, but the amount of power available is limited due to the
environmental effects of damming rivers.
Nuclear fission power accounts for
approximately 20% of the world’s current energy production. However, nuclear fission power plants, in
which heavy atoms are split into lighter atoms, produce inconvenient amounts of
radioactive waste products that are hard to dispose of safely. The supply of uranium-235, the fuel used in
these reactions, is somewhat limited, as well.
Solar, wind, and geothermal energy are some
other methods of creating electricity, but they can not generate nearly enough
energy to satisfy demands.
Even if we had enough resources to last
several centuries, one must realize that humanity will probably still exist on
the Earth for thousands of years, if not tens or even hundreds of thousands of
years. As it is, current estimates have
us running into an energy crisis in mere decades – ten to forty years.
3
Solution
The solution to humanity’s need for
electricity is the nuclear fusion reaction.
Nuclear fusion is a nuclear reaction in which two nuclei are joined
(fused) to create a larger nucleus.
There are several atoms commonly involved in fusion experiments:
Deuterium (D): an isotope of hydrogen containing one
neutron in addition to the proton.
Deuterium is fairly common in water, with about one in 6000 hydrogen
atoms in water being deuterons.
Tritium (T): another isotope of hydrogen, but with two
neutrons. Tritium does not occur
naturally, as it has a half-life of 12.3 years, but tritons can easily be bred
from lithium, which is quite abundant.
Helium-3 (He3): an uncommon isotope of helium with only one
neutron instead of two.
Helium-4 (He4): the common form of helium, with two protons
and two neutrons.
Litium-6 (Li6) and lithium-7 (Li7): Lithium-7 is the more common of the
two. About 7.5% of lithium is
Li6. Lithium is common in minerals and
seawater.
Neutrons (n) and protons (p):
the nuclei of atoms are made of these particles. Protons have a postive charge, while neutrons are neutral in
charge.
The Deuterium-Deuterium Reaction:
D+D ® T
(1.01 MeV) + p (3.02 MeV)
or
® He3 (0.82 MeV) + n (2.45 MeV)
Each
reaction is equally likely.
The Deuterium-Tritium Reaction:
D+T ® He4
(3.5 MeV) + n (14.1 MeV)
Lithium Reactions:
n+Li6 ® He4
(2.1 MeV) + T (2.7 MeV)
n+Li7 ® He4
+ T + n (This reaction consumes some
energy)
(Note:
‘MeV’ stands for ‘million electron volts,’ an eV being equal to 1.60207 × 10-19 J.)
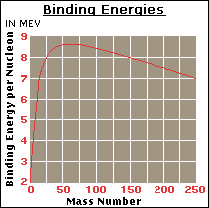 The energy in these reactions comes
from the mass defect phenomenon. Single
protons and neutrons have specific masses (1.007276 u and 1.008665 u,
respectively. A ‘u’ is one atomic mass
unit, which is equal to 1.66054 x 10-27 kg). However, when the mass of an atom’s nucleus
is determined experimentally, it is less than would be expected by merely
taking the sum of the masses of the correct number of individual protons and
neutrons. The amount of energy lost by
the atom can be calculated using Einstein’s equation E=mc2. As can be seen in the graph, starting from
the lightest atoms, the binding energy (a measure of the mass defect of a
nucleus) per nucleon (proton or neutron) of an atom goes up as the mass number
goes up, until it begins to descend at nickel-62. To the left of Ni62, fusion reactions release energy; to the
right, fission reactions release energy.
The energy in these reactions comes
from the mass defect phenomenon. Single
protons and neutrons have specific masses (1.007276 u and 1.008665 u,
respectively. A ‘u’ is one atomic mass
unit, which is equal to 1.66054 x 10-27 kg). However, when the mass of an atom’s nucleus
is determined experimentally, it is less than would be expected by merely
taking the sum of the masses of the correct number of individual protons and
neutrons. The amount of energy lost by
the atom can be calculated using Einstein’s equation E=mc2. As can be seen in the graph, starting from
the lightest atoms, the binding energy (a measure of the mass defect of a
nucleus) per nucleon (proton or neutron) of an atom goes up as the mass number
goes up, until it begins to descend at nickel-62. To the left of Ni62, fusion reactions release energy; to the
right, fission reactions release energy.
Nuclear fusion is actually quite common in
the universe. Unfortunately for our
electricity needs, it is only common in environments such as the centres of
stars.
For fusion reactions to occur, the nuclei of
two atoms must collide and ‘stick’ together.
However, the nuclei of atoms have positive charges due to their protons,
so when two atoms approach, they repel each other because of the like
electrical charges. This repulsion must
be overcome by extremely large forces in order to get the particles close
enough for the strong nuclear force to bind them together. In the Sun, this is done by the huge
gravitational compression force that the Sun’s mass generates. The sun used up most of its easily-fused
deuterium long ago, however, so now fuses hydrogen atoms (p) into He4 (it
actually goes through a series of reactions in order to get to He4, as it needs
to change two protons to neutrons by positron emissions).
We can not create gravitational forces here
on Earth strong enough to cause fusion, so we must find other ways to cause
fusion to take place.
 The
two most common schemes for initiating fusion are the tokamak reactor and inertial
confinement.
The
two most common schemes for initiating fusion are the tokamak reactor and inertial
confinement.
Tokamak Reactors
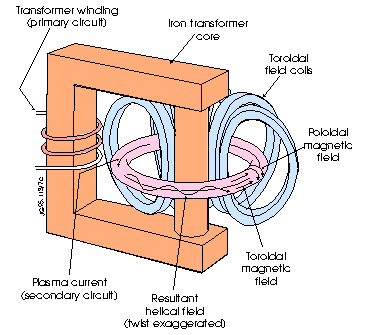 The tokamak reactor was
invented by Russians Igor Tamm and Andrei Sakharov in about 1950. “Tokamak” is a contraction of Russian words
meaning “toroidal chamber-magnetic.” It
is a toroidal (doughnut-shaped) vacuum chamber. A very small amount of deuterium and tritium gas is pumped into
the chamber, then is heated enough to become plasma. Plasma is the fourth state of matter, in which atoms are stripped
completely of their electrons. To
prevent the plasma from touching the walls of the reactor and cooling, large
magnetic coils, as illustrated in the diagram on the next page, induce a
toroidal magnetic field to contain the plasma.
To heat the plasma further, a current is induced in the plasma by the
transformer. This is called Ohmic
heating, as it is the resistance that generates more heat. This current causes poloidal magnetic fields
that ring the band of plasma and help confine it. The toroidal and poloidal fields cause the existence of helical
(spiraling like a Slinky) field lines which the electrons and nuclei spiral
around. As the temperature rises,
resistance in the plasma is reduced to a point where Ohmic heating is no longer
useful. High-energy deuterons and
tritons are now introduced into the tokamak in what is called Neutral-Beam
Injection. These atoms ionize into
plasma and add their energy to the plasma, heating it further. When the plasma has reached approximately
100 million ºC, fusion reactions begin producing energy. Unfortunately, we are currently unable to
achieve ‘ignition’ -- that is, reaction rates that produce the necessary amount
of energy to generate a self-sustaining series of fusion reactions.
The tokamak reactor was
invented by Russians Igor Tamm and Andrei Sakharov in about 1950. “Tokamak” is a contraction of Russian words
meaning “toroidal chamber-magnetic.” It
is a toroidal (doughnut-shaped) vacuum chamber. A very small amount of deuterium and tritium gas is pumped into
the chamber, then is heated enough to become plasma. Plasma is the fourth state of matter, in which atoms are stripped
completely of their electrons. To
prevent the plasma from touching the walls of the reactor and cooling, large
magnetic coils, as illustrated in the diagram on the next page, induce a
toroidal magnetic field to contain the plasma.
To heat the plasma further, a current is induced in the plasma by the
transformer. This is called Ohmic
heating, as it is the resistance that generates more heat. This current causes poloidal magnetic fields
that ring the band of plasma and help confine it. The toroidal and poloidal fields cause the existence of helical
(spiraling like a Slinky) field lines which the electrons and nuclei spiral
around. As the temperature rises,
resistance in the plasma is reduced to a point where Ohmic heating is no longer
useful. High-energy deuterons and
tritons are now introduced into the tokamak in what is called Neutral-Beam
Injection. These atoms ionize into
plasma and add their energy to the plasma, heating it further. When the plasma has reached approximately
100 million ºC, fusion reactions begin producing energy. Unfortunately, we are currently unable to
achieve ‘ignition’ -- that is, reaction rates that produce the necessary amount
of energy to generate a self-sustaining series of fusion reactions.
Proposed Power Generating
Tokamak Reactor
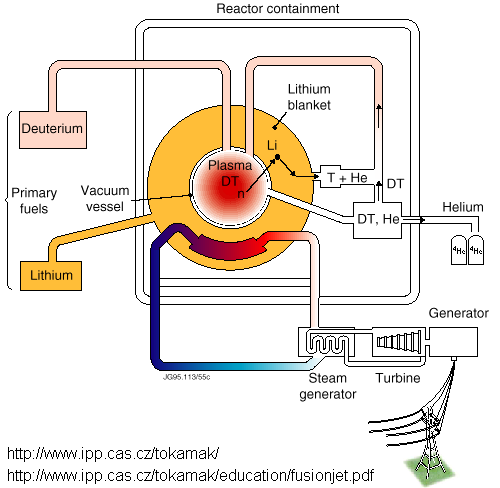
When we have the ability to create a self-sustaining
reaction, the Inter-national Thermo-nuclear Experimental Reactor (ITER) will be
built. It is expected to be 12 m in
diameter and 8 m tall, generate 0.5 to 1.5 GW of power for thousands of people,
and cost at least 3 billion dollars.
ITER will ‘burn’ D-T fuel. The D-T reaction produces neutrons, which
can cause materials to become radioactive.
To slow the reactor from becoming radioactive, the inside has a
‘blanket’ of lithium. High-energy
neutrons react with the lithium, producing He4, T, and often another neutron,
but with lower energy than the original.
The operation cycle of the reactor will be similar to the
following:
-
A small amount of D-T fuel will be injected into a
near-vacuum within the chamber.
-
The fuel is ignited
-
Each burn will last about 20 minutes, during which
time,
-
D-T fuel will fuse into He4, releasing high-energy
neutrons
-
The neutrons (n) and He4 will heat water that is
used to power an electricity-generating turbine. This is only about 35% efficient. Advanced turbine systems that are 50% efficient may be used
instead.
-
Some of the n will react with the lithium blanket,
producing more T, He, and n.
-
Burning is stopped.
-
He4 impurities and leftover fuel are removed from
the chamber (fuel is reused) and the lithium blanket is replenished
The D-D reaction is actually superior to the D-T reaction: it does not slowly make the reactor
radioactive and its fuel is essentially unlimited (our supply of lithium for
the D-T reaction could only last several thousand years). The reason we do not use D-D is that it is
much harder to ignite, as deuterons are smaller than tritons, so if the fuel is
just D, there is less chance of nuclei colliding and fusing.
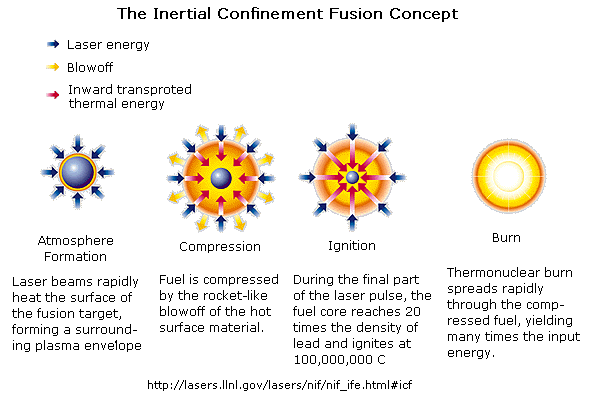
Inertial Confinement Fusion
In inertial confinement fusion, a small pellet of frozen
D-T fuel about 3 mm in diameter is bombarded from all sides with laser or
particle beams, vapourizing the outer layer into a plasma. As this plasma layer tries to expand, it
exerts pressure on the pellet, and the core of the pellet implodes and causes
nuclear fusion.
4 Conclusion
It will probably be 10 to 15 years before we
are able to realize a useful D-T fusion reaction rate, but when we do achieve
this goal, we will immediately have a new goal: the D-D reaction. Every
problem we solve opens a door to another hall of doors. Perhaps one day we will open a door and
there will be no door behind it.
I do not think I would like to have been born
after this last door was opened, as we would have nothing else to strive for.