 YBa2Cu3O7
YBa2Cu3O7Everything is relative and "High-Temperature" Superconductors (HTSC) are no exception. Would you say a temperature of -180ºC is high ?. One hundred and eighty degrees Celsius below the freezing point of water is a hell of a low temperature to most people. But to scientists used to deal with temperatures much lower than that, the discovery of materials presenting superconductivity at those temperatures was hot news.
In the old days (1910-1986) superconductivity was only known for a few metals and alloys and a bunch of ceramic materials. but in all cases critical temperatures were extremely low (10 to 20K, that is -263 to -253ºC)
In general, the structure of
high-Tc superconductors is like a GIANT SANDWICH.
Layers of Copper and Oxygen atoms (the
meat, the essential part for superconductivity) alternate with layers of
other metals (the bread).
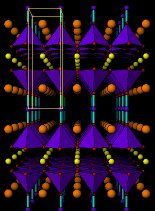
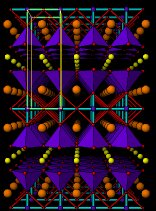
The structure of the 123 superconductor
is shown below. In fact there are two structures: the one on the left corresponds
to the oxide with a smaller content of oxygen (YBa2Cu3O6)
which is not a superconductor. On the right, we have the same structure
with just some more oxygen added (doping) leading to the superconducting
YBa2Cu3O7 In this case the meat
are the planes of Cu-O forming the bases of the blue pyramids and the bread
is also formed of copper atoms (small blue balls with cyan bonds)

 YBa2Cu3O7
YBa2Cu3O7Perspective views of the structures of YBa2Cu3O6 (insulator) and YBa2Cu3O7 (superconductor). Small yellow balls are Y3+ ions, larger orange balls are Ba2+ ions. Small blue balls Copper atoms and small red balls O2- ions. Unit cells are outlined in yellow. Blue pyramids represent the active copper ions coordinated by five oxide ions. Cyan sticks represent bonds between copper ions (in the "bread" layers) and oxide ions.After YBaCuO new and more complex copper oxides have been discovered and higher Tc's have been achieved. In order of increasing Tc we can mention a whole family of Bismuth-containing copper oxides, then Thallium-based copper oxides and finally the series of Mercury copper oxides. These days the record high Tc (130K, -143ºC) is held by the oxide Ca2Ba2Cu3HgO8 , the structure of which is shown below. For comparison we also include the structure of CaBa2Cu2HgO6 , which can be easily compared with that of YBa2Cu3O6 .
CaBa2Cu2HgO6 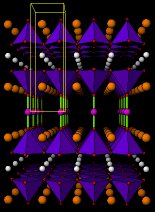
Ca2Ba2Cu3HgO8
Perspective views of the structures of CaBa2Cu2HgO6 and Ca2Ba2Cu3HgO8 (Tc =130K). Small white balls are Ca2+ ions, larger orange balls are Ba2+ ions. Small blue balls Copper atoms, small purle balls Mercury atoms and small red balls O2- ions. Unit cells are outlined in yellow. Blue pyramids represent the active copper ions coordinated by five O2 ions. Green sticks are bonds between Hg ions (in the "bread" layers) and oxide ions.
Last modified: 7 Feb 1999
©Pedro Gómez-Romero, 1998, 1999
![]() Back to main page on Science Technology and Society
Back to main page on Science Technology and Society