 Is
this a Molecule ?
Is
this a Molecule ? Is
this a Molecule ?
Is
this a Molecule ?Benzene is the simplest one. Just one hexagonal array of 6 Carbon atoms, each bonded to two C neighbors and to one Hydrogen. The german chemist Friedrich August Kekule was the first one to realize its hexagonal structure. The following drawings are three different ways to represent a molecule of benzene:
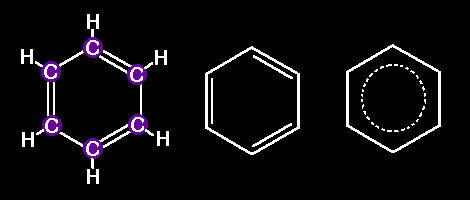
And there are
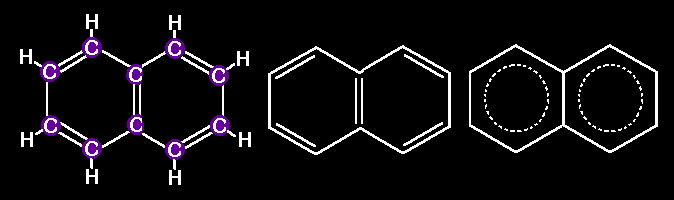
many other larger molecules built with hexagonal arrays of carbons. Look
at this series of linearly fused rings. All of them represent REAL
molecules
|
Benzene
|
 |
|
Naphthalene
|
|
|
Anthracene
|
|
|
Naphthacene
|
|
|
Pentacene
|
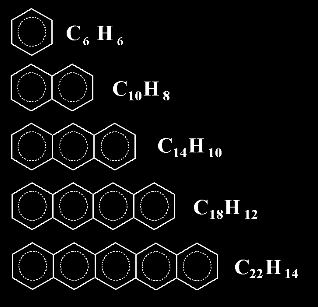
Probably you don't know the name
of the following member of this series, a molecule with 6 fused rings.
It's all right, I don't know it
either. But I bet you could guess its formula !. Could you ?
(Find the
answer at the end of this page)
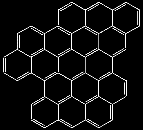
Well, that is not bad. Now, how about putting rings together not only in one dimension to make chains but in two dimensions ?. We might get molecules like the ones below. Again, they ARE ALL REAL molecules
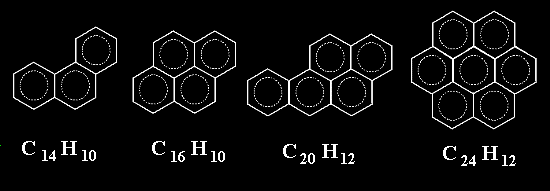
How far can we get with this trick ?.
Well, there is one natural product,
probably not too far from you right now, which is made of just carbon atoms
arranged in hexagonal patterns. It's graphite
and you can find it in the core of your pencils.

A tiny grain of graphite
is made of billions of carbon atoms arranged in layers like the one shown
above. The layers in turn are stacked on top of each other but are not
bonded together, which gives graphite its soft character.
By going from
benzene (liquid) to naphthalene (soluble white solid) to graphite (insoluble
black solid) we have come a long way. Graphite is an extended solid with
properties radically different from those of molecular solids like naphthalene
of anthracene.
For instance,
graphite is an electrical conductor: electrons can go through that material
by jumping from one C atom to another. Molecular solids, made of billions
of single molecules like the ones shown before, are insulators. Benzene
or naphthalene are compounds with single bonded units (molecules) a few
atoms in size ( 10-10
meters, Angstroms); graphite bonded units extend over much wider dimensions
(10-6 meters, microns).
We jumped from one to the other very quickly, but... what is in between
?.
The nanometric dimension.
Are there molecules
with say 100 C atoms ? or 1000?.
How small can
we break a tiny particle of graphite?.
Could we get
a 100 000 atoms chunk?. Could we isolate a single layer ?
How could we
make a linear polymer of fused rings of formula C40002H20004
?
Would that polymer
be an electronic conductor ?
All those are open questions.
The fact is that
we are just beginning to appreciate the importance of molecules, compounds
or solids of nanometric dimensions. The discovery of fullerenes is just
one example of startling new discoveries in this direction (see the related
story in Hot Molecules: Fullerenes). After all,
scientists have only been playing with all the fancy compounds now found
in Organic Chemistry textbooks for just five generations. There is still
plenty to be done and plenty to be found... in the nanometric dimension.
-------------------------------------------------
The sixth member of the series would be C26H16
.
In general, the nth member of this linear series, with n
rings, would be C4n+2H2n+4
Last modified: 1 Mar. 1999
©Pedro Gómez-Romero, 1998, 1999
![]() Back to main page on Science Technology and Society
Back to main page on Science Technology and Society