|
| LinkExchange Member | Free Home Pages at GeoCities |
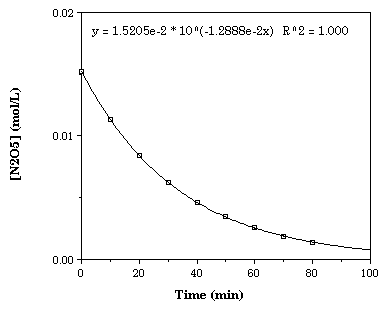
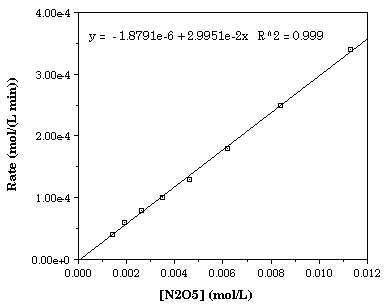
First Order Kinetics
|
| LinkExchange Member | Free Home Pages at GeoCities |
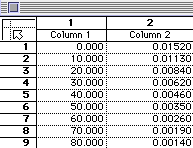
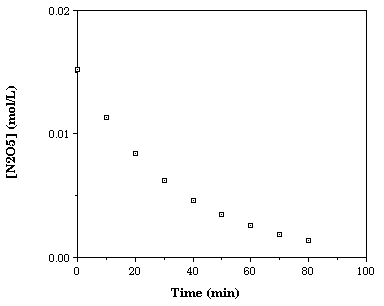
| Time (minutes) | [N2O5] (mole L-1) |
| 0 | 0.0152 |
| 10 | 0.0113 |
| 20 | 0.0084 |
| 30 | 0.0062 |
| 40 | 0.0046 |
| 50 | 0.0035 |
| 60 | 0.0026 |
| 70 | 0.0019 |
| 80 | 0.0014 |
 Return to Master Alchemist Index
Return to Master Alchemist Index