previous page
next
page
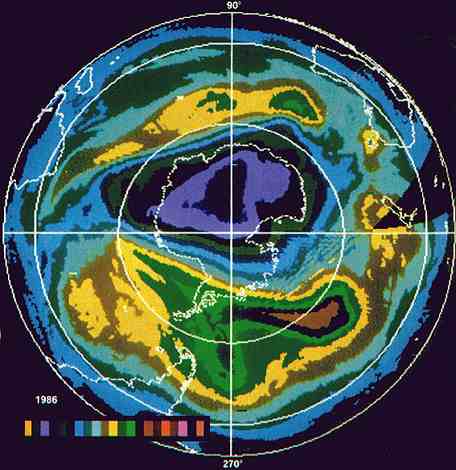

THE OZONE ENIGMA
What's causing a hole over the South Pole in the atmosphere's
ozone layer?
A NOXIOUS form of oxygen, ozone impairs vision and breathing
when it occurs in smog. But in the upper atmosphere, 12 to 30 miles above
the ground, it protects life on earth by intercepting the sun's damaging
ultraviolet radiation. During the past eight years this protective layer
of ozone has become thinner each spring over the South Pole, as seen in
these images (left) from the Nimbus 7 satellite. From 1979 to the present,
a hole (shaded dark purple) has deepened, within which ozone concentraffons
have falLen by 40 percent. A color bar (above) identifies concentrations
from lowest, at left, to highest, at right. Some scientists believe the
ozone was attacked by chlorine released by chlorofluorocarbons, widely
used industrial chemicals. Others theorize that the ozone was destroyed
by nitric oxide produced in the atmosphere by the sun during an active
solar cycle, or that the ozone was pushed aside by upwellings of air from
lower levels of the atmosphere. Whatever the cause, the potential effects
could be serious: If the ozone continues to disappear, skin-cancer incidence
could rise sharply.
A result of natural activity, or man's? So far science has more questions
than answers.
The North Pole, on the other hand, at times resembles a turn-of-the-century
coal town. For in winter, when the Arctic is tilted into constant night
and the sun cannot generate cleansing winds and precipitation, the largest
single mass of pollution sits atop the globe like a dirty cap.
Wearing bulbous boots and mittens as thick as down comforters, I rode
with two technicians to the Barrow Observatory in Alaska. Atop stark huts
where we looked at solar-radiation instruments, the wind reached down the
tunnel of my parka hood to stab my cheeks.
It was early November, and at 8 a.m. a full moon still shone like a
bright dime, and the aurora borealis looped a ghostly scarf across the
sky. By late November the sun would not rise at all. When it reappeared
in late January, it would shine on a haze of sulfates and soot that would
remain until spring storms flushed them out.
Eight nations touch the Arctic -- the United States, the Soviet Union,
Canada, Finland, Iceland, Norway, Sweden, and Denmark (Greenland). Who
soils it? Air masses are mixtures of numerous gases and particles unique
to their places of origin. The Arctic pollutants showed a "signature" unknown
to Western scientists.
Dr. Kenneth Rahn of the University of Rhode Island found they included
arsenic, selenium, antimony, and indium, in a combination that pinned most
of the pollution to a mineral-rich smelting area in the Soviet Union's
Ural Mountains. Other investigators found dry-cleaning Freons and degreasing
solvents used commonly by the Russians but rarely by Western nations.
This method of tracing pollution to its source sprang from the ability
to examine smaller and smaller samplings-now as little as one part per
trillion parts of air.
Tracing pollutants is becoming a political necessity because air is
no respecter of boundaries. On the acid rain issue alone the Scandinavians
are angry at the British, the Canadians are impatient with the United States,
and the Northeast blames the Midwest for dead trout and dying trees. From
the accused the answer has become a familiar refrain: "You can't prove
that my emissions are killing your. . . ."
With fingerprinting and tracking of particles with lasers,
the disclaimers become less convincing. But the dying continues, and we've
made the ammunition for these killing fields all too available, says meteorologist
Volker Mohnen, who gathers cloud data from atop Whiteface Mountain in upstate
New York.
"Water droplets in clouds become little chemical converters,"
he explained. "In a normal ecologically balanced system, ammonia from decaying
matter is present in the air along with natural oxides of sulfur and nitrogen.
Ammonium sulfate or ammonium nitrate are therefore created, falling to
the ground to become natural fertilizer.
"That's the regular cycle-living things on the ground die, decay, and
release ammonia that nurtures more living things. What we are now doing
is to inject additional sulfur, nitrogen, and hydrocarbons into the atmosphere,
altering the cycle."
IF THE MECHANICS of acid rain formation are becoming better understood,
the effects are less clear. Something is killing trees, but scientists
are finding that the deeper they look, the more complex the picture. There
are multiple theories about forest damage:
Dr. Bernhard Ulrich of the University of Göttingen in West Germany
first sounded an alarm when his tests of German soils in the early 1970s
showed high acid inputs from the atmosphere. The result, he predicted,
would be forest damage as acids leached nutrients from leaves and soils
and as trees pulled aluminum and heavy metal ions into their systems. Both
aluminum and heavy metals are present but immobile in many soils, but acid
solutions mobilize them.
The role of prophet might have seemed due the energetic, almost sprite-like
biologist had not a blizzard of other theories emerged. Damage to ponderosa
pines by ozone had been demonstrated near the Los Angeles area in the U.S.
in the 1960s, and the same pollutant has been embraced by many as a major
cause in Germany.
Others felt that air pollutants merely weakened trees so they could
be killed by drought and pathogens. Excess nitrogen from emissions of nitrogen
oxides also gained popularity, on the theory that trees rich in nitrogen
were not hardening for protection from winter.
Problems in pinning the culprit stem from our own basic ignorance of
the environment. Time and time again I was told by scientists on both sides
of the Atlantic that the search for causes of forest dieback has shown
how little we know about how trees grow.
"There is no generally accepted step-by- step process demonstrating
that a pollutant affects trees," said Dr. Arthur Johnson, soil scientist
at the University of Pennsylvania. "We just don't know enough about the
exact mechanisms of tree growth to do that. The only pollutant on which
there is a consensus as to its injuring trees at a distance is ozone, in
the case of California's ponderosa pines and in white pines in the East."
Searching historical records covering more than a century, the University
of Pennsylvania team has found consistent red spruce dieback following
either an extremely warm summer or a cold winter, with a combination of
the two being especially deadly. Perhaps, Dr. Johnson says, trees weakened
by climate variations are then finished off by air pollution.
"That's why you see so little action on controls right now, because
it's such a complex picture," said Volker Mohnen, the White-face Mountain
cloud chaser. "Should we reduce the emissions by 10 percent, 20 percent,
or 50 percent to correct problems not fully understood? This would constitute
a major interference with human activities." And human pocketbooks.
next page

