 Forecast
from a clouded crystal ball
Forecast
from a clouded crystal ball
 Forecast
from a clouded crystal ball
Forecast
from a clouded crystal ballINVOKING STORMS of numbers, scientists use computers to simulate how our planet might respond to an expected doubling of carbon dioxide. Their work, based on mathematical models originally designed for long-range weather forecasting, suggests that the earth will warm significantly in the coming century, bringing many changes in regional temperature and rainfall patterns.
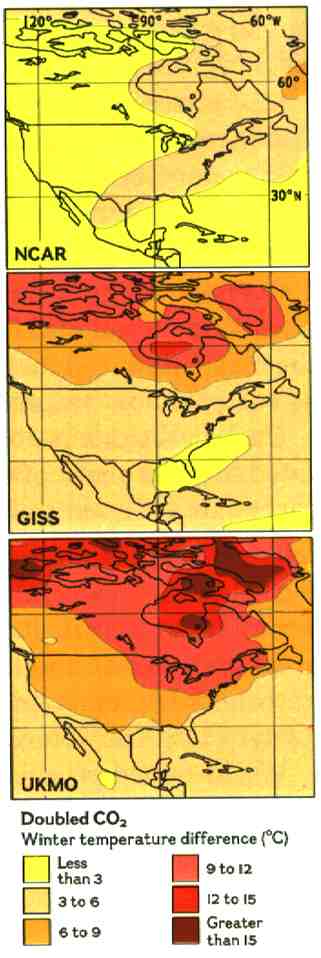
What changes exactly? So far that's impossible to say. As complex as the models are — only a few of the world's supercomputers can run them — they remain rough representations of reality. Moreover, because the models use different methods, their regional predictions vary widely.
Three of the climate models differ markedly as to how North America will warm. Current models do agree on the high probability of warming, and with it major — and unforeseeable — climatic changes. Because the models perform well in simulating seasonal variations and climate over thousands of years, most scientists take their overall predictions seriously.
ANOTHER NOTED climate modeller — and spokesman for potential trouble — is James Hansen, director of NASA' s Goddard Institute (GISS) in New York City . During the scorching hot, dry summer of 1988 he captured international attention when he testified before a Senate subcommittee. "The world is getting warmer," he said bluntly. "We can state with 99 percent confidence that current temperatures represent a real global warming trend, rather than a chance fluctuation. We will surely have more years like this — more droughts and many more days above a hundred degrees — in the 1990s."
He repeated these predictions in subsequent scientific meetings and climate symposiums — upsetting colleagues who felt he should hedge his concerns with more qualifications and maybes. But that is not Jim Hansen's way.
Some have heard and heeded what he and others have been saying. Senator Albert Gore, Jr., of Tennessee, one of the most outspoken politicians calling for action in the face of world warming, has said bluntly: "The greenhouse effect is the most important environmental problem we have ever faced. [It threatens] loss of forests, widespread drought and famine, loss of wild species... topsoil, stratospheric ozone. ... Do we have the capacity, the will, to change habits of millennia in a generation?" Stephen H. Schneider of NCAR (right), an intense, curly-haired prophet of the future, has been deeply involved in climate research for more than 20 years. He writes, speaks, and travels incessantly; he is one of the worried scientists to whom policy-makers listen carefully.
"I agree with Jim Hansen and others that the world has gotten warmer over the past century — faster than ever since about 1975," he told me. I'm not so quick to say it' s entirely due to the greenhouse effect — though that's certainly there. Natural climate variations may be at work too, reinforcing — or at other times masking — the greenhouse forcing. In coming decades some years and some parts of the world may be cooler, but others will be much warmer than normal.
"The 1988 drought in North America, for example, has been linked by colleagues at NCAR with the El Niño phenomenon of the tropical Pacific Ocean. A shift in the jet stream, caused by massive ocean-atmosphere interactions in the Pacific, was the likely cause of that hot, extremely dry summer. "But whatever the local and temporary weather changes, the world can't wait for proof of warming before trying to do something about it. We're engaged in a huge experiment, using our earth as the laboratory, and the experiment is irreversible. By the time we find the greenhouse warming has damaged earth's ability to feed its people, it will be too late to do much about it."
What would he have us do about it now! Try to slow the release of greenhouse gases — by more rigorous energy conservation and changes in fuel use (natural gas releases half as much CO2 as coal); by reducing the burning of rain forests, which increases the level of CO2 in the air; and by planting many more trees, wherever possible.
"Keep in mind, it's not only carbon dioxide that's at fault," Steve Schneider points out. "Methane, which is released from decomposing tundra and marshes, rice fields, termites, and the guts of cattle, is increasing in the atmosphere faster than CO2, at something like one percent a year. And molecule for molecule it has 20 to 30 times the greenhouse effect of CO2. Nitrogen gases, from fertilisers as well as car exhausts and factory smokestacks, chlorofluorocarbons (CFCs) and other industrial products — all the other gases we're pouring into the air — are already doubling the warming potential of CO2 alone.
"We can hope to reduce some of them, such as the CFCs that attack stratospheric ozone," he said. "But the others go with the industrial development of the world; all we can realistically hope is to slow their release, to gain time to cope with the results. "If we can delay a 2°C increase of global temperature from 2025 to 2050, we will have more time to develop alternate energy sources: Nuclear – possibly fusion – is one option, despite its problems. But tapping the sun directly, by solar heat plants and converting sunlight directly into electricity, is both possible and coming down in cost."
I was to see his future in California, at the Rancho Seco nuclear plant outside Sacramento, flanked by 20 acres of solar panels slowly swinging with the sun across the sky; at a pioneering solar-cell factory near Los Angeles, where a breakthrough in "thin-film" technology was closing in on conventional electric power costs; and on a treeless mountain pass above Oakland, where a seemingly endless array of propellers taps the sun's energy from the winds sweeping in off the Pacific.
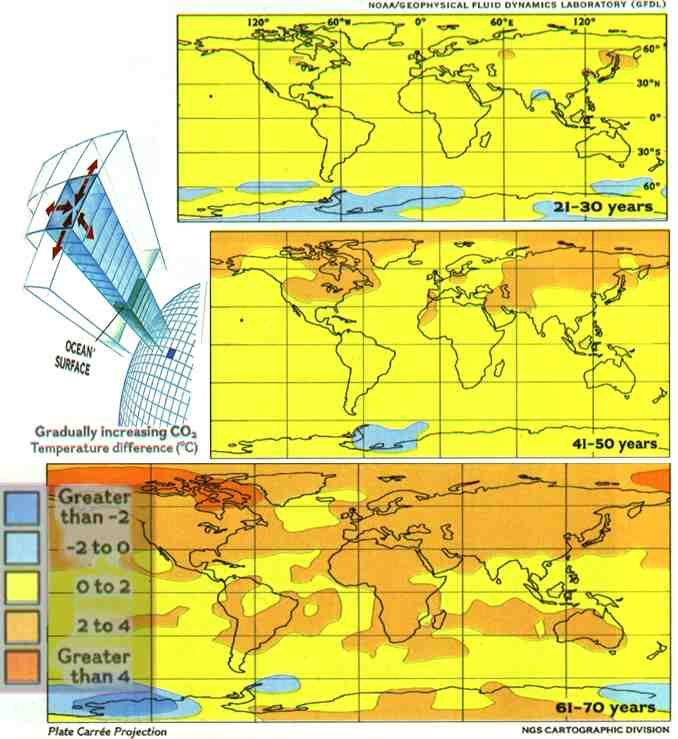 Rather
than programming a computer simply to double CO2 levels, recent models
attempt to track change over time, more accurately depicting the gradual
way heat-absorbing gases accumulate. Researcher'; at the Geophysical Fluid
Dynamics Laboratory told their supercomputer to increase CO2 by one percent
a year from a starting date of 1958. After 30 computer years global surface
temperature had risen by an average of 0.7°C. (Remember that these
are computer simulations and do not give results identical to what actually
happens in the real world.) After 50 simulated years, the temperature had
risen by 1.4°C. And after 70 years, when CO2 had doubled, it had risen
by 2.1°C (3. 8°F). The biggest surprise: The waters around Antarctica
failed to warm as expected. These models reveal new patterns, clues that
scientists may soon be able to check against field data to see how their
predictions hold up.
Rather
than programming a computer simply to double CO2 levels, recent models
attempt to track change over time, more accurately depicting the gradual
way heat-absorbing gases accumulate. Researcher'; at the Geophysical Fluid
Dynamics Laboratory told their supercomputer to increase CO2 by one percent
a year from a starting date of 1958. After 30 computer years global surface
temperature had risen by an average of 0.7°C. (Remember that these
are computer simulations and do not give results identical to what actually
happens in the real world.) After 50 simulated years, the temperature had
risen by 1.4°C. And after 70 years, when CO2 had doubled, it had risen
by 2.1°C (3. 8°F). The biggest surprise: The waters around Antarctica
failed to warm as expected. These models reveal new patterns, clues that
scientists may soon be able to check against field data to see how their
predictions hold up.
To manage their data, modellers divide the earth 's surface into
boxes. Typically each box is about 300 nautical miles on a side and is
sliced into layers of atmosphere and ocean. Based on average conditions
within each layer, such as wind, temperature, sunlight, soil moisture,
and relative humidity, the computer calculates how internal processes will
affect the surrounding boxes. As the calculations are modified again and
again, weather patterns emerge. Simulated seasons change just as real seasons
do.
COMPUTER FORECASTING of climate is uncertain for reasons other than the sheer complexity of the equations. There are variables and feedbacks that even the best of the models barely approach. The oceans are the chief reservoir of heat, controlling weather over the entire globe. As currents such as the Gulf Stream carry the heat from the tropics to high latitudes, cold water from the polar regions sinks and flows toward the Equator, overturning the seas about every thousand years. "The tropical oceans are the driving mechanism of the climate," says climatologist Eric J. Barron of Pennsylvania State University. "The oceans are the memory of the climate system," adds Kirk Bryan of GFDL at Princeton. Yet until recently even the most advanced mathematical models treated the oceans only as vast, shallow swamps.
Carbon dioxide is absorbed by seawater, some of it incorporated into the shells of tiny marine creatures that die and become carbonate sediments on the bottom. Scientists estimate that a significant part of the seven billion metric tons of carbon released into the air each year is taken up in the seas. Oddly, the colder the water, the more CO2 it can hold. As the oceans warm under the effect of more CO2 in the atmosphere, there is great uncertainty about how much of that new CO2 will be absorbed. Is there a limit to how much carbon can be locked away? Have the oceans already reached their holding capacity?
World-famed geochemist Wallace S. Broecker of Columbia University's Lamont-Doherty Geological Observatory worries that rapid switches in ocean circulation might occur under
relatively small changes in global climate. Ice and seafloor core samples show that there have been sudden climate changes in the past, he says, from warming conditions to marked glaciation and back in as little time as a century. It could happen again.
AS THE SEAS AND AIR WARM, more water evaporates into the atmosphere, creating more clouds and another great enigma to the mathematical modellers. Much is yet unknown about the net effects of clouds on global weather. "Clouds are the window shades of the planet," says Steve Schneider of NCAR. "They may be even more important than the oceans or the greenhouse gases in regulating the heat received from the sun."
In daylight low thick clouds reflect sunlight back into space and have a cooling effect. At night they hold in heat radiated from the surface and thus warm the atmosphere. High thin clouds, such as cirrus, may act differently, also adding to the greenhouse effect. Storm clouds transport and release vast amounts of heat.
The incredibly complicated interactions of the atmosphere, land, and oceans lead many scientists to doubt that local and regional weather patterns can ever be accurately predicted for more than a few days into the future. Listening to atmospheric physicists discuss the new mathematical science called chaos is a form of mental mugging; they speak of random walks, strange attractors, and climatic ripples such as the "butterfly effect" – the notion put forward by MIT meteorologist Edward Lorenz that the flap of butterfly's wings in Peru could lead to a tornado in Kansas. Yet these are today's frontiers of understanding.
The limit of that understanding leads Eric Barron of Penn State to quote Mark Twain's famous droll remark: "The researches of many commentators have already thrown much darkness on this subject, and it is probable that, if they continue, we shall soon know nothing at all about it."
Feedbacks – the relationships between the natural forces that control climate – will be the crucial key, most modellers agree: clouds affecting surface temperatures; rainfall and droughts changing soil moisture, vegetation, and evaporation; snow and ice melting from ice caps and glaciers, changing the reflectivity of the planet and raising the level of the seas.
More volcanic eruptions, throwing fine dust and gases high into the stratosphere, might operate against the greenhouse, cooling the earth temporarily. But the best computer models suggest that to bring on marked cooling, volcanic explosions far more violent than those of Mount St. Helens in 1980 or Krakatoa in 1883 would have to occur every five years for as long as a century. The resulting dirty air and acid rain would be worse for life on earth than global warming. If a return to ice-age conditions rather than greenhouse warming sounds farfetched, it was thought a serious possibility as recently as the mid-1970s. The nine major interglacial periods of the past million years have each lasted scarcely 10,000 years before the cold returned – and it has now been longer than that since the last great continental ice sheets melted back. And even though global temperature has been rising since the start of the industrial age, from 1940 until 1970 it leveled and even declined slightly in the Northern Hemisphere.
J. Murray Mitchell, Jr., senior climate researcher of the U. S. Weather Bureau and later of NOAA' s Environmental Data Service, was one of those who documented that downward drift. Now retired, he told me recently: "We thought natural forces, such as volcanic activity or perhaps variation in the sun's radiance, might be at work. But we still don't know whether it was a real change or just a quarter-century-long twitch in the climate cycle."
DOES THE SUN BLAZE absolutely uniformly, sending always the same amount of light, heat, and other radiation into space? Is its total radiance constant, as has long been assumed, or does the energy received by the earth vary, even minutely? The question is crucial in today's climate studies.
From astrophysical evidence the sun is thought to have been 25 to 30 percent dimmer when the earth was young-three and a half billion years ago. Pondering how life could have developed under this "faint young sun," earth scientists postulate that a super-greenhouse effect must have been at work, with 100 to 1,000 times as much CO2 in the atmosphere. Otherwise the surface of the planet would have been frozen solid, and photosynthesis impossible. Yet it indeed occurred, absorbing much of the carbon dioxide and producing the oxygen in the atmosphere necessary for the evolution of life.
In the time of modern science the sun's radiation has seemed absolutely steady. Astronomers have tried for more than a century to detect any change in the "solar constant." It was only in the past decade that they succeeded.
The answer lay in taking solar instruments above the unsteady window of earth's atmosphere, into the black clarity of space. That goal was reached in February 1980 with the launch of the Solar Maximum Mission (SMM) satellite, dubbed Solar Max. It went into space to read solar output just as the number of sunspots — the dark areas on the sun's face that signal changes in its magnetic activity — had reached a peak in their 11-year cycle.
By 1985 Solar Max showed a real, though very slight, decline in the sun's brightness. The drop was only about one-tenth of one percent, but to solar physicists such as Richard Willson of the Jet Propulsion Laboratory, a principal scientist of the project, it was startling. If there is an actual fluctuation of the sun's output of even that small amount, it might have a long-term, measurable effect on global weather.
In 1986 the number of sunspots reached a minimum, as predicted. Shortly after, there began a rapid increase in sunspots — greater than in any previous solar cycle of this century.
Scientists expected the upturn to continue until the next peak in 1990 or '91, but an unforeseen hazard put the Solar Max project in peril. As the sun's activity increased, it warmed the outermost fringe of earth's atmosphere slightly, causing it to expand. The added drag began to slow the satellite, and it dropped in its orbit by a few kilometers. Instead of circling the planet at least until 1991, Solar Max began tumbling in August 1989, and by early December ended its life as a fireball of blazing metal in the sky.
EVEN WITHOUT the Solar Max readings — long before this century, in fact — we've known that changes occur in the sun," I heard from John A. Eddy of the Office for Interdisciplinary Earth Studies in Boulder. He is one of the world's leading solar historians. "Chinese, Korean, and Japanese court astronomers recorded spots on the sun at least 2,000 years ago. Galileo saw them in his first telescope in 1610. The fact that the spots varied on a regular cycle wasn't recognised until 1843, by a German amateur astronomer, Heinrich Schwabe. Their number and position changed, and in some years there were more of them, in other years and decades, many fewer.
"We know today," Jack Eddy went on, "that the spots not only are real but also indicate massive changes going on in the sun. As the spots cross its face — moving with the rotating body — they affect the total energy the sun sends out into space.
As outward evidence of magnetic disturbances on the sun, the spots sometimes herald solar storms or flares, which can disrupt short — wave radio communications on earth or with satellites and cause destructive surges in high-voltage power networks. In March 1989 a massive solar flare disrupted the electric-power grid of much of eastern Canada and produced spectacular shimmering lights in the ionosphere. The pulsating red, green, and white curtains called the aurora borealis, or northern lights, were seen as far south as Florida and Texas.
DO SUNSPOTS affect the planet's weather and climate? There have been times in past centuries when sunspots were very scarce — or missing entirely, if lack of any record of them can be believed. One notable period was between 1645 and 1715, the so-called Maunder Minimum, named for a British solar astronomer of the 19th century.
The coincidence of their absence with the particularly cold period of the Little Ice Age, which gripped Europe from the 1400s to the 1800s, has long intrigued solar scientists. Eddy and other astro-physicists point to the Maunder and a sequence of earlier minimums as the clearest evidence of long-term change in the sun's total activity, perhaps in cycles longer than the principal periods of 11 and 22 years, and of a possible connection between sunspots and the earth's climate.
The evidence remains circumstantial. "But there can be little doubt," Eddy has written, "that variability is a real feature of the sun. The challenge now is to understand it." One clue to sunspots and their effects on the earth lies in an unlikely repository — the record of weather changes locked in the growth rings of trees. At the Laboratory of Tree-Ring Research of the University of Arizona at Tucson, director Malcolm Hughes and others showed me the 8,500-year consecutive tree-ring record acquired by that pioneering laboratory in decades of work in the U.S.Southwest. Periods of faster and slower growth in tree rings since the 17th century have been linked to wet periods and droughts — and possibly to the sunspot cycle.
"There is clear variability in much of this tree-ring record, in a pulse close to 20 years, " Hughes said. "Scientists such as Murray Mitchell and my colleague Charles Stockton see this pulse as a combination of the sunspot cycle and a lunar cycle of 18.6 years and relate it to cyclical droughts in the West, such as the 1930s Dust Bowl.
"More than that, varying amounts of a carbon isotope in the tree rings – carbon 14 – may be a clue to long-term changes in solar radiation and its effect on the earth's atmosphere, " Hughes told me. "The irregularities in the carbon-14 production rate are known as the Suess wiggles, for Hans E. Suess, their discoverer. They are extremely important in calibrating and correcting the carbon-14 calendar used to date ancient events from remnants of organic materials, such as ancient wood or bones." Other theories of sunspot-climate relationships have come and gone, but no true "smoking gun" had been found – until the mid-1980s. Then a German atmospheric physicist, Karin Labitzke of the Free University of Berlin, together with Harry van Loon of NCAR in Boulder, published a remarkable fit between reversing winds in the stratosphere, polar air temperatures, and the sunspot cycle. If their discovery is confirmed, it will indicate a direct link between sunspots and the atmosphere of earth – a possibly crucial connection. The work has been cited as among the most significant now being pursued at NCAR.
One connection may be a better understanding of the ozone hole in the so-called polar vortex over Antarctica each winter, a giant whirlpool of stratospheric winds.
IN THE MID-1980s the world became suddenly aware that the protective ozone shield in the atmosphere was in danger – was, in fact, greatly depleted in a huge "hole" over the frozen wastes of Antarctica. The mysterious stuff called ozone, which until then was known to the public chiefly as an acrid, lung-burning element of smog in overcrowded cities, was being destroyed in the stratosphere by chemicals made and released in the 20th century by humans.
Ozone is a variant form of oxygen – the most life-sustaining gas of all. Under the intense ultraviolet bombardment from the sun at the upper reaches of the earth's atmosphere, normal two-atom molecules of oxygen are split into single atoms – O rather than O2, in chemists' terms. Some of these single oxygen atoms rejoin with O2 molecules to form ozone – O3. The amount in the stratosphere is very scant, less than ten parts per million (at sea level the layer would be about as thick as a pane of window glass), but that layer is enough to stop most of the sun's dangerous ultraviolet rays from reaching the earth's surface 10 to 30 miles below.
The possibility of ozone destruction by man-made chemicals had been predicted as early as 1974 by two farsighted researchers, F. Sherwood Rowland and Mario J. Molina at the University of California at Irvine.
Certain industrial gases dubbed CFCs – chlorofluorocarbons – are so highly stable and inert that they do not react with other substances in nature. Thus they have long been used as the coolants in refrigerators and air conditioners, as the propellants in aerosol cans, in making foam-plastic objects such as coffee cups and fast-food containers, and as solvents for cleaning electronic circuit boards and computer chips. But there could be great danger, warned Rowland and Molina, when those same long-lived gases drift to the upper layers of the atmosphere.
In that same region where ozone is created by solar bombardment, the CFCs could break apart, they postulated, freeing chlorine atoms that could attack and destroy ozone molecules by the billions. If this were to deplete the ozone layer around the whole world, it would put all mankind at risk.
The hazard was judged serious enough for the United States to ban CFCs from aerosol cans in the late 1970s. But CFCs are still produced for other uses, and millions of tons more lie waiting to be freed from scrapped refrigerators and air conditioners. Then came the first startling report by British scientists in 1985 of an Antarctic ozone hole, and a rash of scare stories blossomed in the world's press. Emergency field studies of the stratosphere above Antarctica were mounted by U.S. science agencies, led by NASA, NOAA, and the National Science Foundation. In 1987 an ER-2 aircraft, capable of flying to 70,000 feet in the stratosphere, and a DC-8 jammed with instruments flew from Punta Arenas, Chile, near the tip of South America, out across the ice-locked Antarctic continent.
The hole was real; the ozone had dropped by 50 percent. Its destruction was confined within the rotating swirl of winds in the polar vortex. And it was caused by a chemical reaction, not some unfathomed atmospheric phenomenon. The reaction seemed to occur in the presence of thin polar ice clouds that form in the intense cold of late winter, just before the sun returns to strike the polar latitudes. Less than a year later, in September 1987, more than 40 nations sent delegates to Montreal, Canada. The industrialised countries agreed to reduce production of CFCs by 50 percent by 1998. A June 1990 revision called for a 100 percent ban by the year 2000, with a ten-year time lag for less developed nations.
Does another ozone hole develop over the Arctic in its winter? If the Northern Hemisphere, far more populous than the Southern, is also being depleted of its ozone umbrella, it might pose a far more serious emergency. The same team of atmospheric scientists and computer experts, including Robert Watson of NASA and Adrian Tuck and Susan Solomon of NOAA, spent 45 cold, bleak days in January and February 1989 in the North Sea port of Stavanger, Norway. There the same ER-2 and DC-8 flew 28 missions, from the northernmost airstrip that could safely be used, to take readings from the air of the polar Arctic.
It took a year to analyse all the data. In March 1990 the scientists published their answer. The polar vortex and ice clouds existed also in the northern stratosphere, though not to the same extent as in the southern. Ozone was being depleted in the Arctic as well, by as much as 15 to 17 percent at some altitudes. Over the heavily populated mid-latitudes of the globe, the researchers believe, winter ozone levels may have dropped in the past decade by as much as 4 to 6 percent. And even if all CFC production worldwide were to be halted – an unlikely possibility even to the signers of the Montreal Protocol – the amount already existing and waiting to be released to the atmosphere would mean a continuing ozone drop for decades to come.
THE WORRY IS that stratospheric ozone forms the earth's principal shield against dangerous ultraviolet radiation from the sun. This short wavelength light, below the range of human visibility, kills many forms of life — bacteria, for example, which is why it is used for sterilizing surgical instruments and protecting many foods. But ultraviolet also kills beneficial forms of life, and it can affect the life cycle of many plants, both on land and in the seas.
Middle and long wavelengths of UV cause not just tanning and extreme sunburn in human skin but the most prevalent forms of skin cancer. They also can cause cataracts in the eyes and injure the immune responses of skin, which protect us from many harmful, even deadly diseases. The Environmental Protection Agency issued a risk assessment in 1987, predicting that for every one percent drop in global ozone, there would be a one to three percent increase in skin cancers. Global ozone has dropped at least 2 percent in the past ten years, EPA said, leading to possibly four million added cases of skin cancer. In the past ten years alone, dangerous skin cancers have risen by 50 percent. Because of the long latency periods after exposure, doctors believe that case numbers will escalate even faster in coming decades,
AS THE PUBLIC and bodies politic become ever more aware of these issues and hazards to our home planet the fundamental question remains: What can be done to safeguard our future? Much is being proposed, both in this country and in international conferences and discussions among heads of state. Some scientists worry that not enough is yet known about the atmosphere and climate systems to justify spartan proposals of sacrifice and denial. Others counter that something must be done before it is too late.
One ambitious effort is the International Geosphere-Biosphere Programme (IGBP). In 1992 it will begin a 10-to-20-year study of the planet, a massive, coordinated follow-up to the historic International Geophysical Year of the mid-1950s. In this country, planning has been taken on by the National Academy of Sciences and a federal committee whose members include NASA, NOAA the Environmental Protection Agency, the Departments of Energy, Agriculture, and Defense, the National Science Foundation and other agencies. Each has its own projects, such as NASA's Mission to Planet Earth, a proposed 15-to-20-billion-dollar program to study the planet from space platforms to be launched beginning in 1998. The President's budget for fiscal 1991 includes an additional billion dollars for pursuing such research efforts.
NASA's chief scientist for global change, Ichtiaque Rasool in mid-1989 cited to me some of the basic hard facts on which the world's climatologists largely agree:
The trace gases in the atmosphere — carbon dioxide, methane, nitrous oxide, CFCs — are rising rapidly. They are already at the highest levels of the past 160,000 years. These gases incontrovertibly alter the earth's radiation balance through the greenhouse effect. Average global temperature has risen about one degree Fahrenheit in the past century, though not steadily. But will this unusual warmth continue? Or will cooler-than-normal seasons or years return? And what is "normal"?
It is the central uncertainty about natural versus man-made forces in the climate system that causes scientists and politicians alike to hedge their concerns. It is tempting to ask: If nature will correct what we do to the atmosphere, must we give up our profligate ways?
As evidence mounts, the answer seems increasingly clear: We are wild card in nature, in our ever increasing numbers. "Humankind has become a more important agent of environmental change than nature," Frank Press, president of the U.S. National Academy of Sciences, said bluntly at an international meeting of the Group of Seven industrial nations in Paris in mid-1989.
And what we are doing to the earth's atmosphere, to the blue planet on which we live, is not merely ominous.
It may already be beyond correction.