




MEMPHIS, Tenn. – July 16, 2007 – Medtronic, Inc. (NYSE: MDT) announced today that it has received U.S. Food and Drug Administration (FDA) approval to market the PRESTIGE® Cervical Disc, the first artificial disc commercially available in the U.S. for use in the neck.
The PRESTIGE Cervical Disc gives some patients who are suffering from degenerative disc disease which can cause intolerable neck and/or arm pain, the potential for motion at the treated level, as well as another option for pain relief and function. Typically, a traditional motion-limiting spinal fusion procedure is indicated for these patients.
“As an active mom and an amateur triathlete, the PRESTIGE Disc gave me back my life,” said Stacey Brickson, a patient enrolled in the clinical study from Madison, Wis. “I had so much pain and disability from an auto accident that I could not even lift my head off a pillow before I got the PRESTIGE Disc, and I was too active to even think about a spinal fusion,” she added.
As part of the approval conditions, Medtronic has agreed to conduct a seven-year post approval study to evaluate long-term safety and effectiveness. Medtronic is also going to perform a five-year enhanced surveillance study.
In the largest study ever completed in the cervical spine involving 541 patients, results showed that the PRESTIGE Cervical Disc had superior outcomes in neurological success, as well as overall success, a measurement that includes several safety and effectiveness outcomes, when compared to spinal fusion. The study also showed equivalent Neck Disability Index measures and fewer revision surgeries for patients who received the PRESTIGE Cervical Disc. Post-operative examination of PRESTIGE Cervical Disc patients showed that they had a statistically superior overall neurological success rate at 24 months.
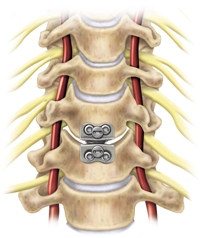
“The patented ball-and-trough of the PRESTIGE Cervical Disc is designed to permit motion at the treated level,” said J. Kenneth Burkus, M.D., an orthopedic surgeon at the Hughston Clinic in Columbus, Ga. “Now patients suffering from cervical degenerative disc disease (DDD) have an alternative to motion limiting spinal fusion.”
The PRESTIGE Cervical Disc is designed to maintain motion and flexibility while replacing a diseased disc that is removed from a patient’s cervical spine. Currently, the most common form of surgery for treating cervical DDD is an Anterior Cervical Discectomy and Fusion (ACDF). More than 200,000 cervical procedures are performed each year to relieve compression on the spinal cord or nerve roots and to implant an interbody graft and metal plate to rigidly fuse the vertebrae together.
The PRESTIGE Cervical Disc underwent a prospective, multi-center, randomized clinical trial to assess its safety and effectiveness based on comparisons between data collected from patients with single-level symptomatic cervical DDD at one level between C3-C7. The investigational group of 276 patients received the PRESTIGE Cervical Disc device while the 265 patients in the control group received an anterior plated surgical fusion utilizing bone graft and plate stabilization.
More information about the PRESTIGE Cervical Disc can be found at http://www.necksurgery.com/ or http://www.prestigedisc.com/.
About The PRESTIGE® Cervical Disc
The PRESTIGE® Cervical Disc is an artificial disc replacement that gives patients suffering from the symptoms of degenerative cervical disc disease or acute unresolved cervical disc herniation a chance to maintain their pre-operative physiologic function and preserve motion in their necks.
The PRESTIGE® Cervical Disc offers patients with radiculopathy and myelopathy related to degenerative cervical disc disease (DDD) in the cervical spine an alternative to spinal fusion surgery. Constructed of stainless steel in a unique, two-piece ball-and-trough configuration, the device is designed to preserve spinal mobility and alignment at the treated vertebral segment.
Each vertebra in the spine is separated by a shock absorbing disc, which is made up largely of water. As discs lose water content because of disease, injury or age, they compress, or lose height, which causes the vertebrae to move closer together. This reduces the disc's shock absorbing qualities, which may lead to bone spurs and narrowing of the nerve openings. If a disc ruptures, it can place pressure on the surrounding nerve roots and the spinal cord, resulting in pain, numbness and/or weakness.
Your doctor may recommend surgery if non-surgical treatment fails to provide relief from these symptoms. Traditionally, a procedure called an anterior cervical discectomy with fusion (ACDF) has been the "gold standard" for surgically treating DDD in the cervical spine. Using bone grafts and instrumentation such as metal plates and screws, this procedure fuses, or creates a bond between, two or more adjacent vertebrae, ideally stabilizing the segment and providing relief. Many patients have achieved excellent results with ACDF; however, a potential disadvantage associated with spinal fusion is the loss of motion and flexibility in the treated vertebral segment.
Artificial disc replacement with the PRESTIGE® Cervical Disc offers the potential for preserved neck mobility at the treated vertebral level.
The PRESTIGE® Cervical Disc replaces a diseased or damaged disc and is designed to preserve motion. Made of stainless steel, the device has two articulating components (a ball on top and a trough on the bottom) that are inserted into the disc space and attached to the vertebral bodies on either side. These components function like a joint, replicating the physiological motion (flexion, extension, side bending and rotation) and alignment (height and curvature) of a natural intervertebral disc.
The PRESTIGE® Cervical Disc is available in a variety of sizes that allow surgeons to closely match a patient's anatomy.
While many factors contribute to the longevity of an artificial disc, the PRESTIGE® Cervical Disc has undergone significant testing to verify the safety and adequate durability of the device.
Read more about PRESTIGE® Cervical Disc clinical research.
Find out if you are a candidate for the PRESTIGE® Cervical Disc.
Talk with Dr. Singer to learn more about the PRESTIGE® Cervical Disc.
It is important that you discuss the potential risks, complications and benefits of the PRESTIGE® Cervical Disc with Dr Singer prior to receiving treatment, and that you rely on Dr Singer's judgment. Only your doctor can determine whether you are a suitable candidate for this treatment.