The Fate of N,N-diethyl-m-toluamide in the Environment
Second Year Oral Proposal
Submitted by Peter Lorey
October 30, 1997
Abstract
The compound N,N-diethyl-m-toluamide, also known as DEET, is the active ingredient in many insect repellents and is the most often used of all mosquito repellents.1,2 It has been concluded by some from available information that using DEET poses a very low risk of causing serious adverse effects.3 However, DEET has been shown to cause severe toxic reactions, and in some cases death, when ingested.2 Literature is filled with studies on the effects of DEET on laboratory animals. However, there is little or no information on the transport
and fate of DEET in the environment. The objective of this proposal is to determine to what, if any, extent DEET is retained in the soil and vegetation of a study area. This will be accomplished by adding 14C labeled DEET to forest plots at the Hubbard Brook Experimental Forest in New Hampshire. The DEET will be synthesized from the 14C labeled starting materials of m-toluic acid and diethylamine. Three replicates of each of three experimental plots and one reference plot will be used, for a total of 12 plots. Each one will be chosen
so that it is 20 m by 20 m in size and contains one tree approximately centered in the plot. The experimental plots will be evenly covered through the use of a backpack sprayer with a solution containing DEET in 95% ethanol. Three dose levels of either 1.25, 12.5, or 125 mg/m2 will be applied to the experimental plots picked randomly. The application will occur in late May. Samples will be collected on a staggered basis on the following days: 1, 2, 3, 7, 15, 30, 60, 90, and 120. This will show both short and longer term pathways. The
samples to be taken will include: soil (top 10 cm), tree branches, tree leaves, groundcover, and carbon dioxide. Two random samples will be taken for each type on each day from each plot. The samples will be analyzed by liquid scintillation counting to determine the 14C activity contained within them.4 The data will be used to help determine whether DEET is degraded, or incorporated into the surroundings.
Introduction
Many people are all too familiar with the annual summertime nuisance of a biting mosquito. Fortunately there are many products designed to prevent people from being bitten. The most common mosquito repellent is N,N-diethyl-m-toluamide (DEET).2 Although it is the most widely used mosquito repellent, DEET is also commonly used in repellents for fleas, biting gnats, sandflies, deerflies, chiggers, and ticks.5 It is believed that DEET interferes with receptor cells in the antennae which bind lactic acid. Lactic acid, which is given off from
warm blooded animals, is known to be a molecule which attracts mosquitoes.6 DEET was developed by the United States Department of Agriculture and first synthesized in 1954.2,7 It is estimated that DEET is applied by 200 million people worldwide annually.7 Many studies have been done on DEET using laboratory animals as test subjects. Information has been gathered on absorption through the skin8, biodistribution9, and metabolism in the liver.10 There have even been a few studies using human volunteers.11 Reilly Industries, Inc., owners of
the world’s largest DEET producer Morflex, Inc., boast of DEET’s safety based on toxicological and dermatological testing. They do, however, caution users that the product is “harmful if swallowed”.12 Much of the current safety information on DEET is based on clinical literature, animal toxicology studies, and the experience of poison control centers. The conclusions from this information indicated an extremely low risk of adverse effects when using DEET.3 However, the cases which included severe reactions or death all involved ingestion,
whether intentional or accidental.2 The resulting acute toxic syndrome includes symptoms such as tremors, seizures, and coma.13 DEET is advertised as resistant to being removed from the skin by wiping, and to removal by sweating or washing off.12 In addition, DEET can accumulate in the brain since it is lipid soluble.2 Because of this, long term effects should be considered in addition to immediately toxic effects. Any potential source of DEET should be considered in assessing safety, especially those with the potential to be ingested. It
is very likely that some DEET will be released into the environment through normal product usage. There seems to be no information available on what happens to this DEET. Thus, the objective of this proposal is to determine the fate of DEET in the environment.
For many organic chemicals at low concentrations, the organisms that degrade them do not assimilate the carbon.14 Therefore, it is hypothesized that if 14C labeled DEET is degraded, the CO2 efflux from the soil will contain an increased amount of radioactivity relative to a reference. If DEET is not degraded, the radioactivity will remain in the soil or be incorporated into some plant material.
Project Design
This project relies on the use of 14C labeled DEET. It will be acquired by synthesizing DEET from 14C labeled starting materials. The location of labeling is dependent on the possible degradation products. Some products include N-deethylated derivatives10 as well as NOx, CO, and CO2.15 Based on the structure of DEET (Figure 1) there are three sections of interest which contain carbon. The first is the ring and methyl group, the second is the carbonyl group, and the third is the ethyl groups attached to nitrogen.
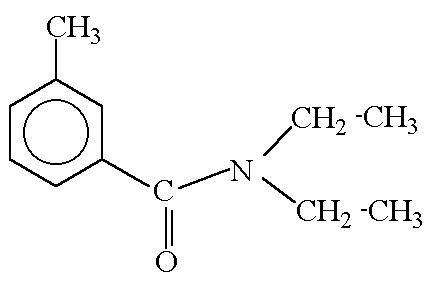
Figure 1. The structure of DEET.
The synthesis outlined by LeFevre1 and Pavia et al.5 will be used. Thionyl chloride is added to m-toluic acid which will have 14C labels on the ring and in the carboxylic acid group. This will give a 14C labeled m-toluyl chloride. This is treated with excess diethylamine which will also be 14C labeled. This gives the final product, which is multiply 14C labeled DEET. The synthesis is shown in Figure 2.
Figure 2. The stepwise synthesis of DEET. Taken from Pavia et al. Introduction to Organic Laboratory Techniques - A Microscale Approach; Saunders: Philadelphia, 1990, p. 369.
The location of the test site is a forested area located in the Hubbard Brook Experimental Forest (HBEF) in New Hampshire (Figure 3). The HBEF was established in 1955 as a center for hydrologic research. The soil, vegetation, and climate at the HBEF are representative of a larger regional area (northeastern U.S. and southeastern Canada). It is forested with deciduous northern hardwoods, and the tree growing season is from May 15 through September 15.16 The nine experimental plots and three reference plots will be sectioned off to include 400
m2 such that each one contains one tree approximately centered in the plot. This is done so that the root system of the tree will be mainly within the plot. The trees will be of the same species, and similar size and age. The reference plots will be kept at a distance from the experimental plots.
Figure 3. Location of the Hubbard Brook Experimental Forest (HBR). Taken from Long-Term Ecological Research in the United States, 6th ed., Long-Term Ecological Research Network Office, Seattle, 1991, p.56.
Since there are no studies of this type, the amount of DEET to add is in question. If too little is added it may be difficult to detect, while too much may give results which are not representative of actual occurrences. There have been studies using organic chemicals to determine their environmental fate and biodegradation products. It has been shown that errors can occur, yielding erroneous conclusions, when extrapolating from the concentrations often used in these studies back to low concentrations.14 Therefore, 50 L of DEET in 95% ethanol
will be made with concentrations of 10, 100, and 1000 µg/ml and applied to the experimental plots. This will give doses of 1.25, 12.5, and 125 mg/m2 respectively. The highest dose, 125 mg/m2 is approximately equal to a 50 ml bottle of 100% DEET being spilled in the 400 m2 area. The use of three different concentrations will increase the chances that at least one will have usable results. Additionally, if all three yield usable results, it will give information on the concentration dependence of the fate of DEET.
Application will be made in late May so that the tree will still be growing for the season. Each level of application will be applied to three of the experimental plots picked at random. It will be sprayed on with a backpack sprayer to give a uniform application rate.
Sampling
Samples will be taken beginning on the day of application. There will be a total of nine sampling dates, which are: day 1, 2, 3, 7, 15, 30, 60, 90, and 120. Thus the sampling will be extended over the course of the summer and will yield information on both short and longer term reactions. The samples to be taken include carbon dioxide from the gas produced in the soil, soil, groundcover (grass), and tree branch and leaves. Two samples of each will be collected from random points in the plot. For wood and other samples which contain cellulose,
approximately 10-25 g will be needed for each sample. Soil samples however, will require approximately 100-500 g since they contain a large inorganic component.4 To minimize contamination, samples should not come into contact with any carbon containing compounds and should be stored in metal containers, typically aluminum, and sealed with a screw top or foil.17 Two random sites in each plot will be chosen and the soil from the top 10 cm will be collected. Likewise, two samples of tree branches, tree leaves, and groundcover will be taken. This
will be repeated for each of the twelve plots.
Sampling for CO2 is less straight forward since concentrations of the gas are dependent upon several factors such as pH, microbial activity, root respiration, and soil moisture and temperature.18 However, the use of reference plots should help to account for variations due to these effects. A rather large volume of gas must be collected in order to obtain sufficient CO2 after other gases are removed. The standard detection method, which will be discussed in the next section, requires approximately 5 g of carbon. This would equate to over 9 L of
CO2 gas. Since CO2 makes up only a small fraction of normal air, a very large amount (over 3,000 L) would have to be sampled. This amount is nearly impossible to handle. However, the need for using very small samples has led to the recent development of small sample systems.4 The method chosen for this project is the one which is most similar to the standard detection method, and will also be discussed in the next section. The use of a small sample system will lower the requirements to around 250 mg of carbon which would decrease the amount of
CO2 gas needed to around .47 L, and the total air sample size would be 156 L. This sample can be collected with the use of Tedlarâ gas sampling bags. These bags are made of polyvinyl fluoride and have nickel-plated brass on/off valves or a silicone septum valve. They have heat-sealed double seams, and are designed for a maximum temperature of up to 250 oF. The largest size available from the Cole-Parmer Instrument Company can hold a volume of 85.7 L.19 Thus, two Tedlarâ bags can be filled and combined to achieve the desired sample volume. Two
samples will be taken from each plot, and the two bags which will be combined for each sample will be located in close proximity to each other. For each bag to be filled, several pieces of capillary tubing will be inserted into the ground. These will be run to a pump which will pump the collected gas into the Tedlarâ bag. Since samples will be taken at intervals of 1,4,8,15, and 30 days, the pump’s flow rate will be adjusted to 60, 15, 7.5, 4, and 2 ml/min respectively in order to fill the bag at an even rate over the entire interval.
14C Counting
The samples will be sent to Southern Methodist University for 14C analysis. The technique used to determine the 14C activity is the same as that which is used to date samples of organic content. The conventional method of analysis detects the decay products of 14C.4 14C decays to 14N and emits a beta particle. It is the beta particle which is detected. The widely used detection method became popular around 30 years ago and is called liquid scintillation counting.4 The first step is to convert each sample to a liquid. The only liquid that is
currently used for the sample is benzene because it contains over 90% carbon and has excellent scintillation properties.4,17 The sample is completely combusted in a closed chamber to form CO2. After purification, the CO2 is then reacted with molten lithium to form lithium carbide. The lithium carbide is then hydrolyzed to acetylene which is then catalytically trimerized to benzene.4 The scintillator is then added to the benzene. The most commonly used is PPO (2,5-diphenyloxazole) although newer ones with improved performance have been developed.17
The scintillator is a molecule which interacts with the beta particle produced from the 14C decay to produce a flash of light. This light flash is detected by a pair of photomultipliers on opposite sides of the vial. The use of two photomultipliers eliminates readings from external radiation which may have gotten into the detection chamber.4 The flash of light is only taken as a true count if both photomultipliers register simultaneously.4
Figure 4. The basic elements of a liquid scintillation counting instrument used for 14C analysis. Taken from Taylor, R.E. Radiocarbon Dating - An Archaeological Perspective; Academic Press: Orlando, 1987, p. 88.
Several methods have been developed recently to handle much smaller sample sizes. These methods include mini-gas counting and low-background liquid scintillation counting.4 The latter is used in this project due to its similarities to the standard liquid scintillation counting which is used for the other sample types. The sample vial is specially designed to hold small volumes, and samples of 250 mg are routinely handled.4 The only drawback in
using a smaller volume is that the counting time must be increased. Counting usually lasts for 960-2760 hours for small sample systems, as opposed to 24-72 hours for standard detection.17 The remainder of the process is the same as for the standard liquid scintillation counting.4,17
Conclusion
As a result of this study, new information will be gained on the fate of DEET in the environment. Whether it is degraded or incorporated into plant material is of interest to many people. Certainly the everyday consumer would want to know if additional hazards exist with the use of this product. It would also be of interest to the manufacturers and other people in the DEET industry to know what happens to it in the environment. If it turns out
that DEET is not totally degraded, it may be necessary to do further studies on it in addition to the animal toxicology studies to include in the safety assessment of DEET.
This project is intended to be more qualitative than quantitative. Based on the results, future projects could be proposed which focus on one specific pathway examined in this project. They could be refined to obtain more quantitative results. Thus, this project will yield new information on the environmental fate of DEET, and could also serve as a starting point for future research.
Literature Cited
(1) LeFevre, J.W. J. Chem. Ed. 1990, 67, 278-A279.
(2) Tenenbein, M. MD. JAMA 1987, 258, 1509-1511.
(3) Osimitz, T.G.; Grothaus, R.H. J. Am. Mosq. Control Assoc. 1995, 11, 274-278.
(4) Bowman, S. Radiocarbon Dating; University of California Press: Los Angeles, 1990.
(5) Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Engel, R.G. Introduction to Organic Laboratory Techniques - A Microscale Approach; Saunders: Philadelphia, 1990.
(6) Weiss, R. Science News 1988, 134, 37.
(7) Castleman, M. Sierra 1995, 80, 24-25.
(8) Reifenrath, W.G.; Hill, J.A.; Robinson, P.B.; McVey, D.L.; Akers, A.; Anjo, D.M. J. Environ. Pathol. and Toxicol. 1980, 4, 249-256.
(9) Robbins, P.J.; Cherniack, M.G. J. Toxicol. Environ. Health 1991, 7, 304-306.
(10) Yeung, J.M.; Taylor, W.G. Drug Metab. Disposition 1988, 16, 600-604.
(11) Selim, S.; Hartnagel, R.E.Jr.; Osimitz, T.G.; Gabriel, K.L.; Schoenig, G.P. Fundam. Appl. Toxicol. 1995, 25, 95-100.
(12) Reilly Industries, Inc. World Wide Web site. http://www.reillyind.com/html/products/data_sheets/c12h17no.html
(13) Verschoyle, R.D.; Brown, A.W.; Nolan, C.; Ray, D.E.; Lister, T. Fundam. Appl. Toxicol. 1992, 18, 79-88.
(14) Alexander, M. Environ. Sci. Technol. 1985, 18, 106-111.
(15) Material Safety Data Sheet for N,N-diethyl-m-toluamide; Fisher Scientific, 1996.
(16) Hubbard Brook Ecosystem Study - Site Description and Research Activities; 2 ed., USDA Forest Service, Northeastern Forest Experiment Station, 1996.
(17) Taylor, R.E. Radiocarbon Dating - An Archaeological Perspective; Academic Press: Orlando, 1987.
(18) Suchomol, K.; Kreamer, D.; Long, A. Environ. Sci. Technol. 1990, 24, 1824-1831.
(19) Cole-Parmer 97-98 Catalog; Cole-Parmer Instrument Company; Vernon Hills, IL, p.10.
(20) Long-Term Ecological Research in the United States, 6th ed., Long-Term Ecological Research Network Office, Seattle, 1991, p.56.



This page hosted by  Get your own Free Home Page
Get your own Free Home Page
