
Response by Jim BaldwinThis is pure baloney. Ozone depletion is cyclic. When Mt. Pinatubo in the Philipines erupted it spued out in one day more that 500 times the amount that man has produced since the production of man-made CFCs (yes, CFCs DO occur in nature). CFC ozone depletion has NEVER been able to be reproduced in a laboratory environment and is only THEORY. Even NASA has stated that they have found no evidence of ozone depletion in their experiments. (No it's not because they want to keep punching holes in the ozone with their launches) Yet, as with Darwin's THEORY of evolution, evolution, has been taught as fact. And we KNOW that all good scientists don't teach theory as fact. It is all part of a catalyst for the new world order and to get the U.S. to cough up more money from it's wealthy coffers to a SOCIALIZED world. There is my "interpretation" your "interpretation" and TRUTH. This is truth.Jim Baldwin USA |
|
"How do we know that natural sources are not responsible for ozone depletion? While it is true that volcanoes and oceans release large amounts of chlorine, the chlorine from these sources is easily dissolved in water and washes out of the atmosphere in rain. In contrast, CFCs are not broken down in the lower atmosphere and do not dissolve in water. The chlorine in these human-made molecules does reach the stratosphere. Measurements show that the increase in stratospheric chlorine since 1985 matches the amount of CFCs and other ozone-depleting substances released by human activities." |
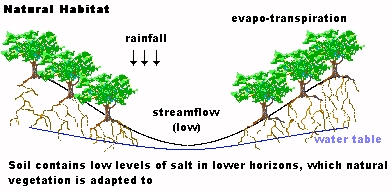
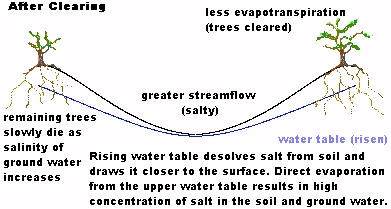
Response by Eric BatemanDan I enjoyed reading your inventive articles on aquaponics etc. It has concerned me greatly about the way Western Australia's growing population puts a heavy demand on water resources and it is terrific to see a local lad offering options on the internet.I remember during the late 50's and into the 60's how water was an issue and similar concerns were expressed about how the (in those days approx 400,000 odd) people would survive on those existing resources without a change of attitude to usage. (I'm 46 by the way.) I was thinking the other day how in the late fifties and into the sixties, Mum would conserve grey water. The way she did that was to run the garden hose into the wash sink and or bath and then drain the 'grey water' into the garden plants. Mostly on the Lemon tree that I remember. Mum would get us kids to connect the hose and hand one end through the laundry or bathroom window, turn the tap on wait a few seconds and then undo our end. Its a pity in our advanced technological society that someone could not invent a simple device that could pump the water from a washing machine or the like into a filtered watering system. I am sure an inventive person could produce such a device. Obviously some big things have taken place. Unfortunately I haven't lived here for over 25 (of 27) years due to a job I took up in Jan 1970 (back Dec 96) so news in the eastern states about development in the WA water conservation area was not of any priority. We came back for two years in 80/81 and I remember grey water was an issue then. I've only just returned and from your article see that the issue is still under discussion without any real community solutions. In your studies perhaps it may provide a thinker like yourself to gear a more urgent approach to debate the issue of WA water and its uses on a much wider scale than the yearly government reference to the problem without any real community solution or initiative except restrictions. Certainly ground water has eased some of the burden but how long will that last? Certainly the recent hot weather spell will make a huge demand on the water supplies not to mention the evaporation percentages. One channel in Sydney during last year informed the viewing public of daily consumption quantities and the dam levels. It had a good community response judging by the figures supplied. I could waffle on for quite a while, so thanks for your page and I will visit again. Eric. |