Colorimetric Analysis of Oxides of Nitrogen and Particulatesfrom the Exhaust of an Internal Combustion Automobile Engine
by WateroseAbstract:The concentration of NOx (oxides of nitrogen) in air samples which were collected from one automobile exhaust was determined by filtration of the particulates and colourimetric analysis of the air samples. The air samples had a mean of 27.8 NOx ppm with a standard deviation of 13.9 NOx ppm at start up and a mean of 5.4 NOx ppm with a standard deviation of 3.7 NOx ppm after idling the automobile engine for 15 minutes. The particulate matter had a mean of 500 mg m-3 with a standard deviation of 346 mg m-3 at start up and a mean of 1300 mg m-3 with a standard deviation of 824 mg m-3 after idling for 15 minutes. Key Words: nitrogen oxides (NOx), particulates, automobile exhaust, colourimetric analysis, internal combustion engine Oxides of nitrogen (NOx) include nitrous oxide (N2O), nitric oxide (NO), and nitrogen dioxide (NO2) (Manahan pp 338-345). The addition of NOx to the atmosphere cause harm by creating smog (Manahan pp 381-405) and depleting the ozone layer (Manahan pp 338-345). Smog causes harm to human health, damage to materials, toxicity to plants, and harm to the atmosphere (Manahan pp 381-405). The sources of NOx are both natural and anthropogenic; natural sources include biological processes and lightening, and, anthropogenic sources include combustion of fossil fuels in stationary and mobile engines (Manahan pp 338-345). The major contributor is internal combustion engines (ibid). The emission standards for automobiles has decreased the permissible amount of NOx and particulates to improve air quality (ibid). The addition of particulates to the atmosphere reduces visibility and causes harm to the human respiratory system (Manahan pp 319-323). High levels of particulates occur with high levels of SO2 and other pollutants; there is a strong correlation between increased mortality rates and elevated levels of atmospheric pollution (ibid). Objectives: The objectives were to compare the amount of NOx and particulates in the automobile exhaust at time of starting the engine and after the engine had idled for 15 minutes. The group sample results were compared to determine the mean NOx , the mean particulates, and the standard deviations for the group sample results. All materials and methods used are as described in the Environmental Science Lab Manual, 1997 for Experiment 7 (Royal Roads University). Air samples were collected from a 1985 Chrysler Laser automobile at start up and after idling the engine for 15 minutes. A group of duplicate air samples were obtained. The absorbance was analysed on a Beckman Model DU-40 Spectrophotometer set at a visible wavelength of 550l . The group results are summarised below in Table 1. Table 1. Batch Results of Particulates and NOx in Exhaust.
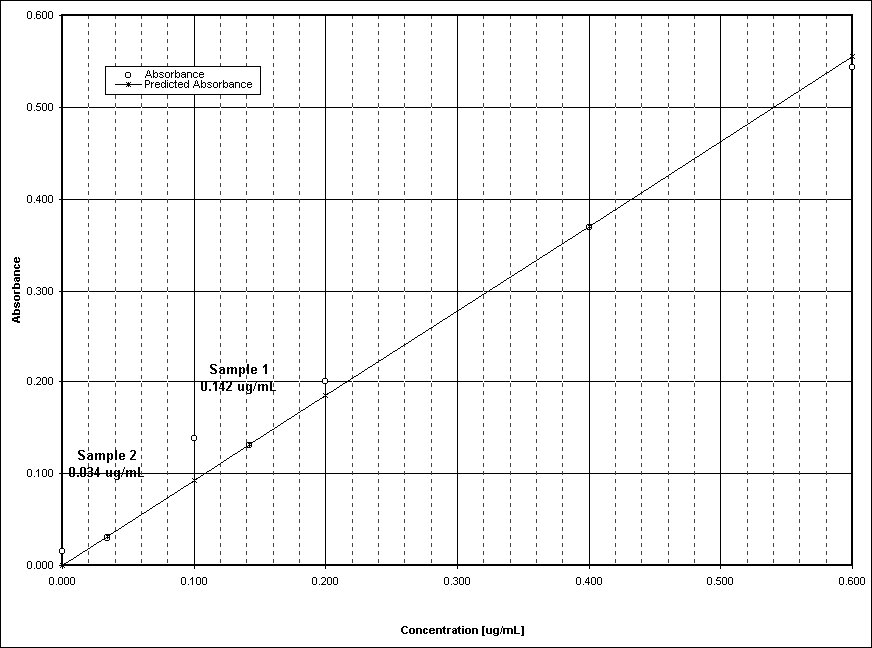
The concentration of particulates in S1 ranged from 200 mg m-3 to 800 mg m-3 , and in S2 from 400 mg m-3 to 2000 mg m-3 . The group average for S1 was 500 mg m-3 with a standard deviation of 346 ppm. The group average for S2 was 1300 mg m-3 with a standard deviation of 824 ppm. There were no visible stains or colours on the white filters after filtering the particulates from the samples for Test 1. The first test (Test 1) contained 800 mg particulates m-3 sample for S1 and contained 2000 mg particulates m-3 sample for S2. The concentration of NOx in S1 ranged from 11.9 ppm to 1 ppm, and in S2 from 1 ppm to 10 ppm. The group average in S1 was 27.8 ppm with a standard deviation of 13.9 ppm. The group average in S2 was 13.7 ppm with a standard deviation of 3.7 ppm. The first test (Test 1) had NOx concentration of 21.1 ppm in sample S1 and NOx concentration of 5.1 ppm in S2.. The NOx concentrations of the samples and the standards for Test 1 are plotted on the calibration curve in Figure 1. Calculations: The calculation of the concentration of NOx was determined by linear regression analysis of the calibration curve. The means for NOx and particulates were calculated by totalling the results of each test and averaging by the number of duplicate sample tests. The standard deviations were calculated by the square of the sum of the difference between the data point and the mean averaged by the number of samples less 1.
There was a wide range in the experimental results of the concentration of particulates and the concentration of NOx . The high range must be attributed to techniques used during the experiment because each test was run on the same engine at the same time using identical equipment. The absorbance readings were obtained sequentially from the same absorbance spectrometer. The only factor that differed between tests was the individual tester; therefore, the differences are likely attributable to technique. A review of literature indicates that the concentration of NOx increases as a function of temperature under conditions found in an internal combustion engine (Manahan pp 338-345). The overall results indicate that the initial concentration of NOx when the engine was cooler at start up was higher than the concentration of NOx when the engine was warmer after idling for 15 minutes. While initially this appears to be opposite to the expected trend the method used to plot the concentration of Nox bears scrutiny. A linear regression was used to determine the concentration of Nox in the air samples. Only two readings were obtained. It is not accurate to plot the Nox concentration based on only two data points. It is possible that the concentration of Nox would have declined over a longer period of time. A more accurate regression could be obtained with a series of readings; a reading taken at one minute intervals extending over a longer period of time. Furthermore, there is an assumption that engine temperature increases over time. In addition, the automobile was stationary during the experiment and the engine did not perform any work other than to maintain the engine at idle speed. The high levels of NOx at start up may be due to an accumulation of NOx after the engine was turned off and started to cool down. The automobile is recognised as a primary contributor of harmful pollutants into the atmosphere (ibid). The automobile industry has taken significant steps to reduce emissions; however, the overall net amount of emissions is likely to increase due to the increasing number of automobiles. In the Vancouver Region, the Greater Vancouver Regional District introduced the Air Care Program to test vehicle emissions and impose maximum emission quantities on vehicles; the maximum emission is specifically rated to the individual vehicle. A command and control approach may temporarily mitigate the problem of poor air quality but this approach does not remedy the long term problem. Ultimately, the long term solution is to replace the internal combustion engine with a non-harmful energy source. A viable alternative is the use of the electric vehicle or development of the Ballard fuel cell vehicle; both alternative energy sources are non-fossil fuel and non-combustible. "It is highly unlikely that the bicycle or the horse and carriage will be considered as a viable alternative for personal transportation in the 20th century...Pity, eh?" [a quote from the Waterose] Manahan, S.E. Environmental Chemistry. Sixth Ed. Lewis Publishers. Boca Raton, Fa. 1994 Royal Roads University. Environmental Science Lab Manual. Victoria, BC. Please be aware that this is a student level lab and has not been peer reviewed by a professional journal. Please refer to primary journal publications for additional information for your research. Student labs are my favourite part of learning because each is a prototype mini-project with multiple stages in: planning, doing, analysing, and writing.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||


 email Waterose
email Waterose
