 | ||||||
| Articles | Projects | Resume | Cartoons | Windsurfing | Paintings | Album |
Industrial Production of Phosphoric Acid
by Waterose

Phosphoric acid, H3PO4, is second only to sulphuric acid in volume produced. It is primarily used for the manufacture of phosphate salts which are used in turn for detergents and fertilisers. There are two methods of production that can be used in its manufacture. The thermal process is used to produce acid from elemental phosphorous. The acid produced is extremely pure however, it is also expensive. The other process used is the wet process. This process manufactures acid from phosphate ores. The majority of phosphoric acid is manufactured using this process.
The Thermal Process:
In this process, phosphorous must be in its elemental form. The elemental phosphorous is burned to produce phosphorous pentoxide (P2O5) which is then hydrated. The heat is then removed and the phosphoric acid (H3PO4) is collected as a fine mist. Due to the high combustion temperatures, the reactivity of hot phosphoric pentoxide, and the corrosive nature of the acid produced, the collection system must be carefully designed. Figure 1 shows the four major collection chamber designs: they are; the wetted wall, water cooled, air cooled, and hydrator-absorber combustion chamber. The design names are based upon the method used to protect the walls of the combustion chambers. The two most popular chambers are the wetted-wall and the air-cooled.In the thermal process, an electric furnace is used to smelt the phosphorous ore using coke and silica. This produces elemental phosphorous which is then burned (oxidized) to become phosphorous pentoxide (P2O5). The P2O5 is then absorbed in water to produce a mist of high quality H3PO4 which is collected. This method yields an extremely high grade product which can be used in food and technical applications. The cost of production is too high for fertiliser use which is manufactured exclusively using the wet process.
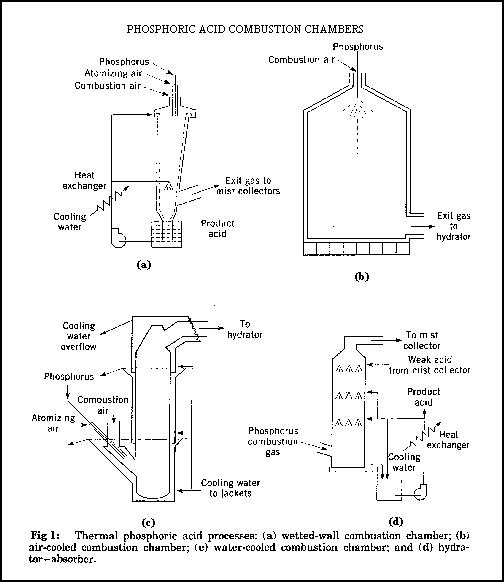
The elemental phosphorous enters the top of the combustion chamber where it is atomised by compressed air or steam and burned. In the case of a wetted-wall chamber, combustion temperatures may exceed 2000°C. This step converts the phosphorous into P2O5. At the base of the chamber, a phosphoric acid spray is located. As the gas stream enters the bottom of the camber, it is hydrated and cooled to 100°C by the spray. Cooling the solution reduces the corrosion to the chamber walls. In a process developed by The Tennessee Valley Authority (TVA), these two steps were separated into different chambers. This allows combustion to occur over a longer period of time which improves the conversion efficiency. After combustion is completed, the gas is blown into the wetted-wall hydrator-absorber where a phosphoric acid spray is used for hydration and cooling. These plants are extremely efficient and produce can produce phosphoric acid up to a strength of 117% H3PO4 (85% P2O5 content).
In air-cooled plants 1-200% excess combustion air is used to reduce the temperature of P2O5 oxidation to 1000-1700°C. The waste heat is disposed of through evaporation is the hydrator-absorber. Within this chamber, the hot gases are cooled by water spray to 100°C. The mixture is bathed in weak phosphoric acid and the strong acid produced is passed through a heat exchanger and collected.
The Wet Process:
The majority of the worlds phosphoric acid production (over 90%) involves this process which produces high volume, low cost, low purity phosphoric acid from phosphate ores. Once produced, acid from this process is used almost exclusively in agriculture as fertilisers and feed supplements. A significant portion is further purified for use in food and technical applications.Sulphuric acid is used to digest phosphate rock (calcium phosphate) producing a calcium sulphate slurry and phosphoric acid, which is separated using filtration. The acid filtrate is continuously recycled to the reactor to concentrate the P2O5 content of the acid produced. There are two temperature ranges that are used to produce phosphoric acid in the wet process. The two temperature ranges will produce phosphoric acid containing different concentrations of P2O5.
At a temperature of 70-80 C.....30% P2O5 content in the liquid phase of phosphoric acid.
At a temperature of 80-90 C.....40% P2O5 content in the liquid phase of phosphoric acid.
Temperatures outside of these ranges result in poor filtration rates and low acid recovery.
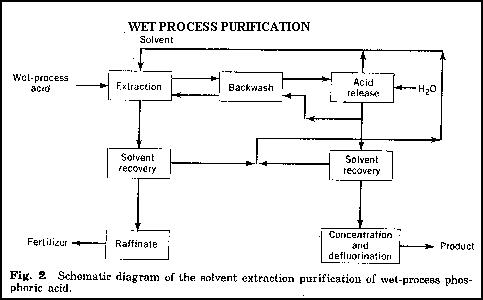
Due to rising electrical costs and declining market demands for technical grade phosphorous acid, the purification of wet process phosphoric acid is becoming more popular in North America. During the purification process, methods such as chemical precipitation, solvent extraction, crystallisation, and ion exchange are used to remove impurities from the acid. Of these, chemical precipitation and solvent extraction are the most popular.
Before purification, the crude acid produced from the wet process is concentrated and clarified. This step provides two benefits; the removal of any remaining sludge and improved partitioning of the acid into the solvent. During this step fluoride is removed through evaporation. An additional step in which precipitates of sulphate, arsenic, and fluorosilicates are removed, is often included prior to purification.
The purification process is based upon the separation and removal of H3PO4 using organic-phase extraction. In this process an organic solvent such as a short chain (4 to 8 carbons) alcohol, ketone, ether, or amine is used. The H3PO4 is removed by passing the crude acid through one or more organic-phase extraction columns using one of the solvents mentioned. This technique extracts from 60-75% of the P2O5 contained in the crude acid and converts it to H3PO4. The residual acid phase containing the remaining P2O5 is referred to as raffinate and is used for fertiliser. The purified acid H3PO4 is then recovered in the backwash step using counter-current extraction. Solvents can be recovered from the raffinate and the purified acid and recycled back into the process. The purified acid can be concentrated to the required P2O5 content.
References:
Written for Royal Roads University ES403 Industrial Processes and Pollution Prevention Lecture SeriesEncyclopaedia of Chemical Technology. 1996. Fourth Ed. Phosphoric Acids and Phosphates. Edited by M.H. Howe-Grant and J.I. Kroschwite. Vol.18. John Wiley & Sons. Pp 669-685.
Return to Index of Articles

 email Waterose
email Waterose
Please Sign My Guestbook
Please View My Guestbook

| Articles | Projects | Resume | Cartoons | Windsurfing | Paintings | Album |
 | ||||||