1) Institute for Neurobiochemistry, The Protein Chemistry Group, Witten/Herdecke University, Germany
2) St. Paul, Minnesota, USA
| A key unresolved issue in the origin of life is the nature of primordial energy conversion. Modern biochemical processes such as photosynthesis, respiration or fermentation are too complex to have emerged during early biological evolution. In contrast, a simple primordial anabolic process termed thermosynthesis has been proposed that makes use of temperature gradients. Thermosynthesis could have played a major role in the origin of life. Even today thermosynthesis may still drive some anabolic reactions in thermophiles living in niches comprising large temperature gradients such as hydrothermal vents. These microorganisms are therefore prime prospects during a search for biochemical relics of primordial thermosynthesis. Here we describe the technical setup and the main strategies to detect thermosynthesis experimentally.
We perform experiments during thermocycling in a conventional thermocycler normally used for the polymerase chain reaction (PCR). Such a programmable apparatus can quickly and repeatedly change the temperature of a 100 microliter solution within a PCR-reaction-vessel in the range between 4 °C and 99 °C (figure 1). For measurements in spatial temperature gradients, PCR-reaction-vessels are placed between a cooled and a heated aluminum support separated by a 1mm slit to obtain sufficient thermal isolation (figure 2). The protein-associated thermosynthesis mechanism (PTS) assumes that a progenitor of contemporary F1, called pF1, had the ability to release strongly bound ATP formed from ADP and phosphate during a thermal unfolding of the enzyme. During a thermal cycle the pF1 thus functions as a heat engine. All that pF1 does is forming a small, dehydrated pocket that binds ADP and phosphate, let these substrates react, and release the ATP formed again (figure 3). |
To detect such an activity within a mixture of macromolecules e. g. within a crude cell extract, newly formed ATP is probably quickly degraded by other catabolic enzymes and thus, not detectable. To overcome this problem, excess of a second enzyme capable to incooperate g-phosphate from newly formed ATP into another molecule is needed. An approach using labeled phosphate, thermophilic hexokinase and glucose is shown in figure 4.
Three experimental strategies to identify possible relics of thermosynthesis making use of the described techniques are currently followed up: 1. Selection of microbial strains, capable to grow in temperature gradients in the absence of other energy sources. 2. Measuring temperature-gradient driven ATP-synthesis in cell extracts of thermophiles or microbial samples taken from environmental niches comprising strong thermal gradients. 3. Measuring temperature-gradient driven ATP-synthesis catalyzed by bioinformatically detected and recombinant expressed thermophile-specific proteins (THEPs) with unknown function. A bioinformatic survey yielded several thermophile-specific proteins (THEPs) with unknown function. THEPs from the hyperthermophile bacterium Aquifex aeolicus were cloned in E. Coli. Aquifex aeolicus is probably one of the oldest bacteria and located close to the root of the phylogenetic 16S rRNA tree of life (figure 5). One candidate protein, aaTHEP1, shows minimal residual ATP hydrolysis activity. Studies to detect ATP synthesis, the reverse reaction, in the presence of temperature gradients are currently in progress.
|

Figure 1: Thermocycler to perform experiments during thermocycling.
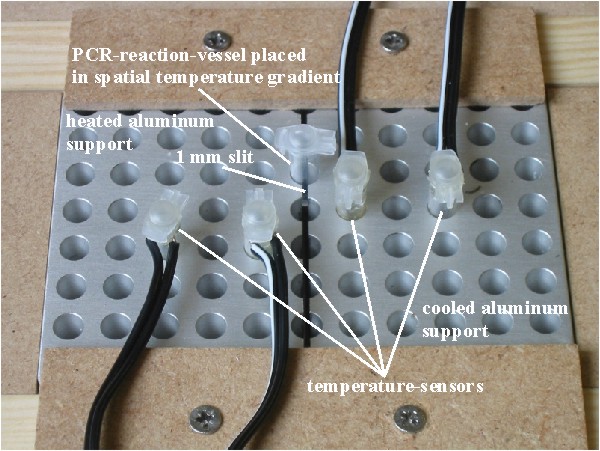
Figure 2: Experimental setup to generate spatial temperature gradients.
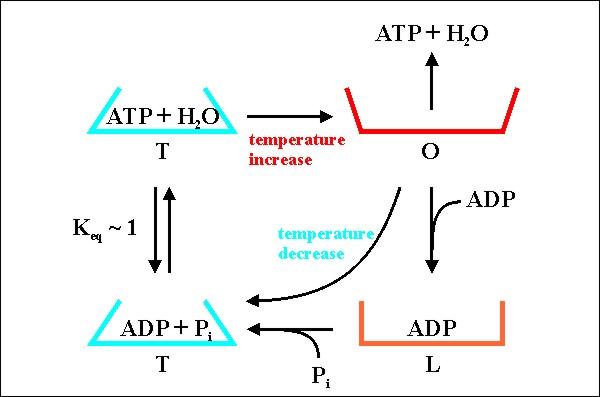
Figure 3: Mechanism of ATP-synthesis catalyzed by a proposed pF1 if exposed to temperature gradients.
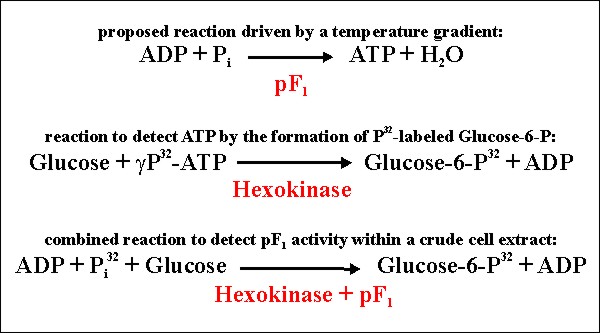
Figure 4: Experimental strategy to detect ATP-synthesis catalyzed by pF1.
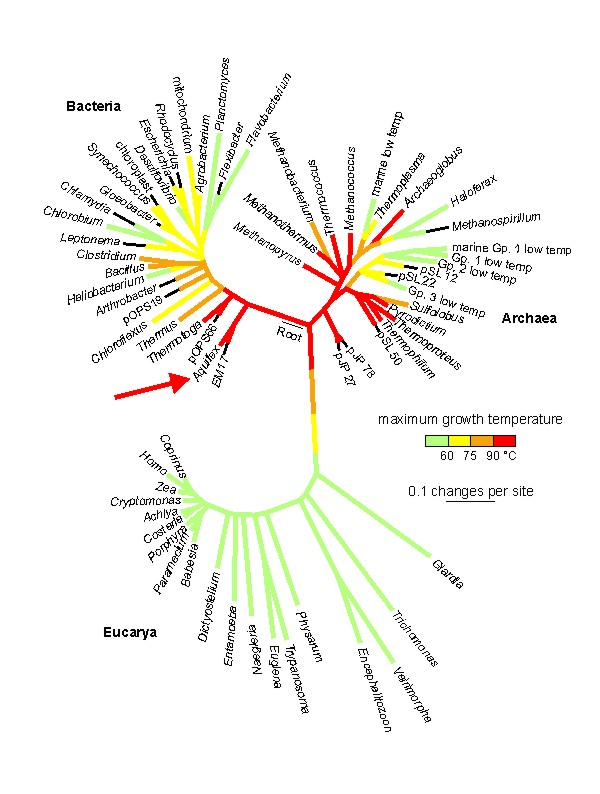
Figure 5: Temperature-coded 16S rRNA phylogenetic tree of life (Lineweaver & Schwartzman). Aquifex aeolicus (arrow) is closely located to the root.