Anthonie W.J. Muller

Thermosynthesis Niches in the Solar System
Department of
Biomedical Sciences
|
Anthonie W.J. Muller |
|
|
Thermosynthesis Niches in the Solar System |
Department of Biomedical Sciences |
Introduction
Present-day biological energy conversion is a complex process. Bacterial photosynthesis (BPS), for instance, uses many components: light-harvesting proteins, reaction centers, charged biomembranes, ATPsynthases driven by protons that move across the membrane. How can such a complex system have emerged during evolution?
The
thermosynthesis theory shows that BPS could have evolved from biological heat engines working on the thermal cycling occuring in convecting volcanic hot springs (Part 1). The earliest energy generating system is a direct progenitor of the contemporary ATPsynthase enzyme. In a simple origin of life model, this first system condenses not only phosphates but also peptides (Part 2). In the resulting polypeptides library a very small fraction of these peptides has itself peptides-condensing capabilities during thermal cycling-the first system propagates. Part 3 correlates the thermosynthesis model with other origin of life studies. The power of thermosynthesis is much smaller than the power of photosynthesis, fermentation or respiration, and it may be difficult to detect. Terrestrial (Part 4) and extraterrestrial (Part 5) environments containing a thermal gradient that could permit thermosynthesis are pointed out.|
|
What is a heat engine? The steam engine is an example of a heat engine. In the boiler the water becomes steam, which pushes a piston, and external work is done. In the condenser the steam becomes a liquid again. This water is returned to the boiler by pumping. The water is thermally cycled. |
|
|
Several types of heat engines are known. In all of them heat enters at a high temperature and leaves at a low temperature. |
part 1. the thermosynthesis model for the evolution of photosynthesis
the chemiosmotic mechanism and bacterial photosynthesis
|
|
the chemiosmotic mechanism In all organisms free energy is carried by the ATP molecule. Almost all ATP is synthesized by the chemiosmotic mechanism.
During photosynthesis and respiration protons are pumped across a membrane. The voltage across the membrane increases. The protons fall back through the ATPsynthase enzyme, which transduces their energy in the free energy of ATP. |
|
|
membrane voltage and proton/ATP ratio ATPsynthase can synthesize ATP, but it can also perform the reverse process, proton pumping across the mem-brane. Whether-at a given membrane voltage-the enzyme functions as an ATP synthesizer or as a proton pump depends on the enzyme's H+/ATP ratio. |
|
|
binding change mechanism
ATPsynthase consists of two parts: Fo and F1. The F1 part binds ADP and phosphate. Upon closing of the enzyme -and in the local absence of water-ATP forms spontaneously. The Fo part transduces the energy of the returning protons by opening F1 (the 'binding change') and forcing the ATP release. |
bacterial photosynthesis
|
|
Proton pumping is a simple process in BPS. Upon photon capture by a light-harvesting complex, the resulting excitations ('excitons') diffuse towards a reaction center. Here the exciton energy is transferred to electrons, which then move along an array of charge carriers to the other side of the membrane, where they reduce a quinone, which alsopicks up protons from the medium. The resulting quinol (QH2) diffuses back across the membrane. The electrons return to the reaction center and the protons are released. |
In the proposed evolution model for photosynthesis the BPS components are removed stepwise: firstly the light-harvesting complex and the quinones (resulting in the PS0 mechanism), next the reaction center (resulting in MTS), and finally the Fo part of ATPsynthase (resulting in the PTS mechanism).
|
|
Instead of generating a membrane voltage by a charge transfer across the membrane, a membrane potential can also be generated by changing its polarization. A voltage difference is present across a dipole layer. |
In the photosynthetic reaction center a large dipole is formed when the excited electron moves to the other side of the membrane. The PS0 mechanism uses the voltage difference caused by the light-induced dipoles in these reaction centers.
|
|
In some bacterial reaction centers the dipole is especially large, as these centers contain a charged stalk that extends far into the medium. In PS0 this stalk has a function, i.e. permitting a large dipole, while in standard photosynthesis this stalk has no function. |
The PS0 mechanism makes use of light/dark cycling. In the BPS machinery the quinones have been removed. The ATPsynthase functions both forwards and backwards during the PS0 cycle.
|
|
All organisms beneath the surface of natural waters are subject to cyclic changes in light intensity due to the focussing and defocussing of the incoming sunlight by surface waves. Photosystem 0 makes use of the fast changes in light intensity that occur beneath the surface of natural waters due to focussing/defocussing of sunlight by waves at the water surface. Such changes cause the complex, fast-moving patterns on the bottom of swimming pools that are shown here. |
|
|
The PS0 cycle. In the light the excited electrons can move within the reaction center, but after having reached the other side of the membrane, they can only fall back in the dark. The dipoles result in a dipole potential that drives ATP synthesis. In the dark the protons are pumped back. |
|
|
The stepwise evolution of the photosynthetic reaction center is easily pictured in terms of PS0. In the very first PS0 photosynthesizer only an intramolecular dipole was present (a), later added charge carriers permitted temporary intermolecular charge separation (b and c), and in (d) the added charge carrier results in charge transfer across the membrane. |
|
|
Lipids can form a monolayer at the water/air interface. Just like liquids and solids, such a monolayer can undergo a phase transition, the 'thermotropic phase transition'. The oriented molecules of the monolayer constitute a dipole layer; the associated dipole potential is easily measured. During the phase transition this dipole potential can change strongly, e.g. can decrease with ~100 mV upon a change in temperature of 5° C. |
A biomembrane can be imagined as consisting of two different monolayers placed back-to-back. Their difference in dipole potentials results in a membrane dipole potential that will change during a thermotropic phase transition. MTS makes use of this change. During thermal cycling the membrane potential varies just as during light/dark cycling in PS0, and ATP can be gained similarly to PS0, using the same ATPsynthase. MTS requires no photosynthetic reaction centers.
|
|
Thermal cycling occurs in convecting volcanic hot springs. |
|
|
The net dipole potential and its change during a thermotropic phase transition. |
|
|
The MTS cycle on a membrane-potential vs temperature plot. |
One can easily imagine ATP synthesis during thermal cycling by F1 only, without involvement of a membrane or Fo, using a variation of the binding change mechanism. Since in the local absence of water ATP is spontaneously formed from ADP and phosphate, all that is needed is a thermal unfolding of F1. This is called the 'temperature-induced binding change mechanism', and the enzyme a 'pF1'.
|
|
The PTS cycle consists of a binding of ADP and phosphate at a low temperature by an unfolded enzyme, followed by its folding, and a spontaneous formation of bound ATP. At a high temperature the enzyme unfolds again, and the ATP is released. The unfolded enzyme is then ready for another thermal cycle.
|
Do the numbers permit this mechanism? The D H for the unfolding of an F1 subunit is ~660 kJ/mol. Assuming a temperature difference D T of 7,5° C, the available free energy according to Carnot equals 660 (7,5/330) = 15 kJ/mol, which is a reasonable value for a phosphate bond (and a peptide bond).
part 2. thermosynthesis applied to the origin of life
During the origin of life, an energy source must have been present before information processing could become possible.
The first living organism arose around an energy conversion system (Granick), which may have been a heat engine (VanHolde).
In a generalization of PTS it is assumed that during thermal cycling pF1 could not only synthesize a phosphate bond, but a peptide bond as well. Just like a phosphate bond, a peptide bond does not require free energy in the absence of water: this absence may be global, but also local, i.e. in an enzymatic cleft. Since pF1 is itself a polypeptide, pF1 could propagate. In the thermosynthesis theory, pF1 is the very first enzyme.
Life starts with an enzyme that resembles the F1 moiety of ATPsynthase, pF1. During thermal cycling pF1 performs dehydration reactions such as peptide bond formation or phosphorylation. After many cycles the resulting daughter protein library contains many different proteins.
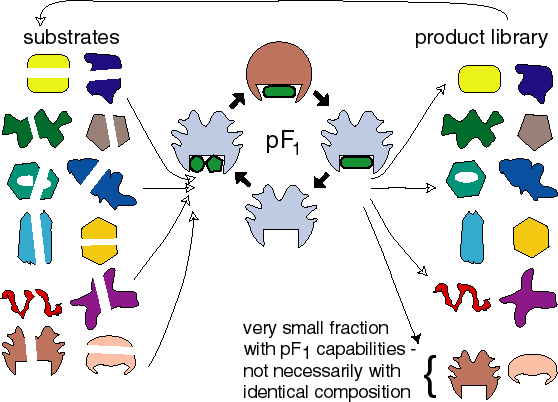
A very small fraction of these proteins can itself function as a pF1. pF1 thus propagates in a functional sense-yielding daughters with the same capability-but not in a compositional sense: the composition of the daughters may differ. The availability of an energy source makes this very inefficient process possible.
In the beginning, a convection current carries the first cell. The contents of a convection cell is thermally cycled, therefore permitting PTS.
The origin of life requires both self-organization and a dissipative structure. A convection cell is one of the few self-organizing dissipative structures known to physics.
|
self-organizing: convection organizes itself: a thermal gradient suffices. dissipative structur e: in the universe structures are either(a) equilibrium structures, i.e. at or near thermodynamic equilibrium; they are at rest, or 'dead', or (b) dissipative structures, i.e. far from thermodynamic equilibrium; these structures can perform external work. Examples are machines and organisms. |
Is the chance formation of a pF1 by a pF1 probable?
Assume a target sequence of 30 amino acid residues: only one in all possible combinations of peptides with 30 residues can function as a pF1.
Note that Avogadro's number NA = 6 1023, and 210 = 1024 ~ 103
20 different residues: chance 1 : 2030 = 230 1030 = (2 10)3 1030 ~ (103)3 1030 = 1039 >> NA ® improbable, but
4 different residues: chance 1 : 430 = 260 = (2 10)6 ~ (103)6 = 1018 << NA ® probable!
Some protein-nucleic-acids (PNA, a progenitor of RNA) enhance the efficiency of pF1 propagation. The very first PNAs may have been purely parasitic, having only the ability of quick reproduction, similar to a virus (Dyson), but later some PNAs were formed that enhanced the pF1 propagation efficiency by functioning as ribosomal PNA, transfer PNA and messenger PNA.
Gradually, pF1 propagation evolved from functional towards compositional, guided by information-carrying PNA molecules. The genetic code evolved until a system with accurate translation capability had been obtained. During or after this process the PNA was replaced by RNA.
part 3. a comprehensive model for the origin of life
Amino acids have been detected in meteorites and have also been observed in Miller's experiments.
Amino acids can form polypeptides when heated:
- proposed condensations in lagoons at low tide at mild temperatures (~50° C) (Bernal);
- demonstrated condensations just above the boiling water of water (~120° C) (Fox);
- demonstrated condensations (which also involved diketopiperazines) at hydrothermal vent conditions (Matsuno).
Upon the addition of proteinoids membranes are formed, 'proteinoid microspheres', with the shape and size of bacteria (Fox)
Convection is a self-organizing dissipative structure (Haken).
In the thermosynthesis theory life emerges upon the addition of proteinoid microspheres to a volcanic hot spring.
Archaebacteria are the last common ancestor of all organisms; they live in volcanic hot springs (Woese).
The chemiosmotic mechanism (Mitchell);
The binding change mechanism for F1 (Boyer; Penefsky);
F1 progenitor pF1; thermal cycling requirement; temperature induced binding change mechanism;
Substrate ambiguity for the first enzyme has been proposed (Black), only one first enzyme may be required: therefore pF1 condenses amino acids, phosphates.
First biogenesis
involved a primitive metabolism (Dyson): in the thermosynthesis theory identified as PTS.PTS capability selects microspheres, as phosphorylation of membrane components stabilizes the membrane-a selective advantage.
Randomly constituted protein synthesis; a minor fraction has itself pF1 capability.
The first nucleic acids may have been parasites that did not give an advantage (Dyson); later some become beneficial, the advantage being guidance of pF1 synthesis.
Ribose problem: where did the ribose in RNA come from? (Shapiro).
Peptide Nucleic Acids (PNAs) have been proposed as RNA progenitors (Nielsen).
The emergence of the system using a genetic code constituted the second biogenesis (Dyson);
The second biogenesis occurs during the gradual increase in accuracy of pF1 synthesis;
The code starts with a codon coding for one amino acid-glycine (Hartman)- with other codons coding for random amino acid insertion;
pF1, first expressed enzyme, 'mother of all enzymes';
proteins ® cells ® enzymes ® genes (Fox);
reverse transcription yields DNA;
genetic apparatus obtained
A lipid, insulating biomembrane is acquired. In general the two constituting monolayers have a different composition: in an asymmetric biomembrane a net surface dipole potential results.
During thermal cycling around the thermotropic phase transition temperature the membrane potential fluctuates.
The emergence of MTS is related to the emergence of ATPsynthase.
In a proton translocator the membrane phase transition partially shields proton carrying groups.
This result in a heat engine that pumps protons during thermal cycling (but does not synthesize ATP) (the pump is not shown: see Muller, 1995).
Upon combination of Fo and F1: variable proton translocation and a variable H+/ATP stochiometry (VanWalraven); net ATP synthesis during thermal cycling.
Photosynthesis in fluctuating light only; light-induced dipoles; increase in dipole size during evolution;
physiological function of the stalk in the reaction center.Upon membrane spanning: reducing power has been obtained.
quinones and quinols; ability of transmembrane diffusion: photosynthesis in constant light;
combined MTS/PS0/PS capability and their association with State 1 - State 2 transitions (not shown);
the Q-cycle (not shown); descendancy from RC; BPS
present day bacterial photosynthesis obtained
Urry (1992): free energy transduction by protein phosphorylation can be explained by a change in the protein folding temperature upon phosphorylation. The effect of Ca2+ upon biomembranes can similarly be interpreted as an effect upon the thermotropic phase transition temperature of the membranes.
If the first organisms lived on thermal cycling, their metabolic processes must have depended on it. The prevalent biochemical processes of regulation by protein-phosphorylation and by Ca2+ can be explained as ways to mimic primordially required thermal cycling isothermally.
This leads to the conjecture:
ATP mimics primordial thermal cycling
which answers the question, 'Why ATP?'
part 4. potential terrestrial niches for thermosynthesizers
The driving force for the evolution from PTS/MTS by PS0 to BPS is increasing power, the free energy generated per unit time. This power is inversely proportional to the characteristic cycle time, which is for
PTS/MTS: the cycle time of the convection current: estimated lower limit, a few seconds, but in general much larger, ~102 s;
PS0: the cycle time of light fluctuations beneath surface waves: 0.1 - 10 s;
BPS: the diffusion time of the quinone across the membrane: ~ 10 ms
The power of BPS will therefore be ~100 times the power of PS0, which in turn will be ~100 times the power of PTS/MTS. One expects any BPS organism to outcompete any PS0 or PTS/MTS organism.
PTS/MTS can, however, work in dark niches: at night, during the polar night, and in subsurface conditions or at great depths in natural waters. It can occur in convection cells, as well as in thermal gradients where an organism can thermally cycle its membranes. An illustrative example is given by chloroplasts in a leaf (which is of course not in the dark!).
|
|
Leaves contain a thermal gradient, as the leaf is warmed at the sunny side and cooled at the shadow side by transpiration. The palissade cells span the leaf-and the thermal gradient-and the protoplasm stream circulates all cellorganelles such as chloroplasts and mitochondria in this thermal gradient, which would permit thermosynthesis in these organelles. |
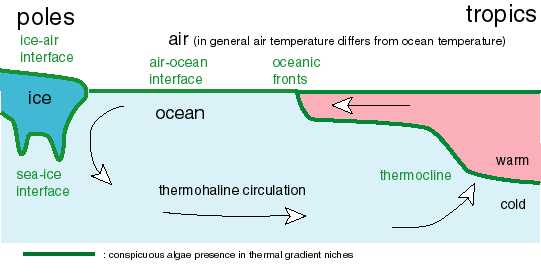
More generally, the protoplasm stream can thermally cycle the cellorganelles in any organism/cell placed in a thermal gradient. The world around us contains many thermal gradients, with a conspicuous organism presence:
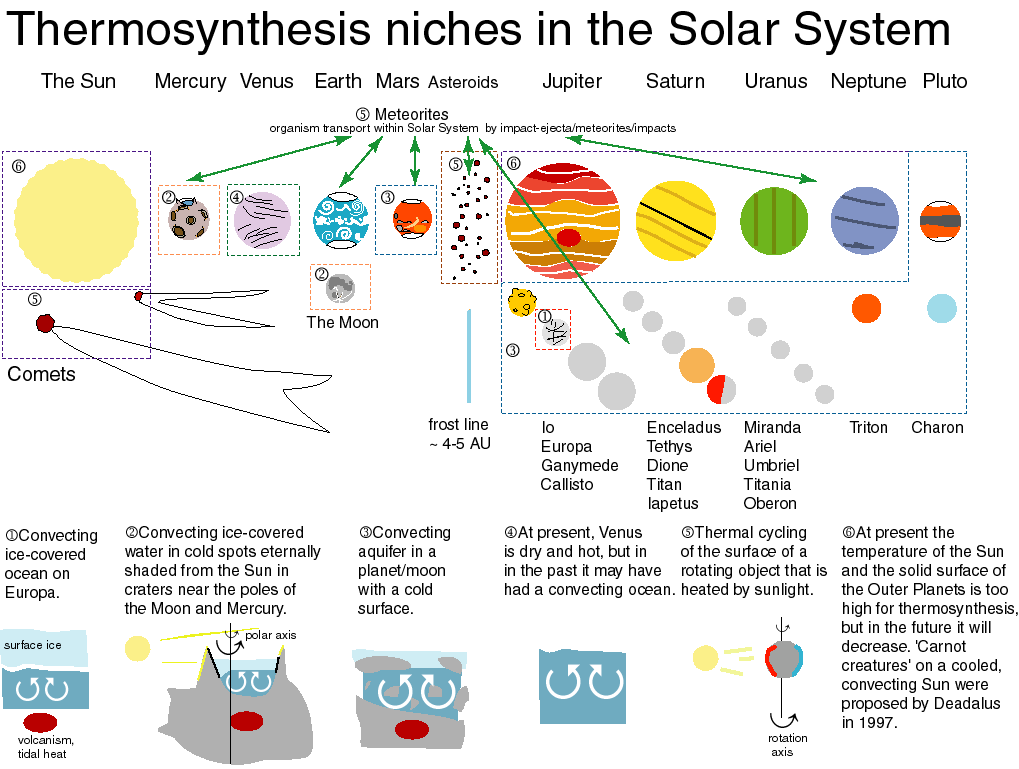
part 5. potential niches for thermosynthesizers in the Solar System
See the Figure.
Summary
Thermosynthesis is not life as we know it - but thermosynthesis life is not that much different.
It provides a simple model for the origin of life,
and the process can occur almost everywhere on Earth and in the Solar System.
Muller, AWJ (1998). Thermosynthesis: where biology meets thermodynamics. In: Uroboros, or biology between mythology and philosophy. pp 139-167 (eds W. Lugowski and K. Matsuno), Wroclaw
Muller, AWJ (1996). Hypothesis: the thermosynthesis model for the origin of life and the emergence of regulation by Ca2+. Essays Biochem. 31, 103-119
Muller, AWJ (1995) Photosystem 0. A postulated primitive photosystem that generates ATP in fluctuating light
Muller, AWJ (1995). Were the first organisms heat engines? A new model for biogenesis and the early evolution of biological energy conversion. Prog. Biophys. Mol. Biol. 63, 193-231
Muller, AWJ (1993). A mechanism for thermosynthesis based on a thermotropic phase transition in an asymmetric biomembrane. Physiol. Chem. and Physics and Medical NMR 25, 95-111
Muller, AWJ (1985). Thermosynthesis by biomembranes: energy gain from cyclic temperature changes. J. Theor. Biol. 115, 429-453
Bernal, JD (1961) Origin of life on the shores of the ocean. In Oceanography, pp. 95-118 (ed. M. Sears), Am. Assoc. Adv. of Science, Washington DC
Black, S (1970) Pre-cell evolution and the origin of enzymes, Nature 226, 754-755
Boyer, PD (1993) The binding change mechanism for ATP synthase-some probabilities and possibilities. Biochim. Biophys. Acta 1140, 215-250
Dyson, F (1985) Origins of life. Cambridge University Press, Cambridge
Fox, SW (1988) The emergence of life. Basic Books, New York
Granick, S (1957) Speculations on the origins and evolution of photosynthesis. Ann. N.Y. Acad. Sci. 69, 292-308
Haken, H (1978) Synergetics. Springer, Berlin
Hartman, H (1975, 1978) Speculations on the evolution of the genetic code. Origins of Life 6, 423-427; 9, 133-136.
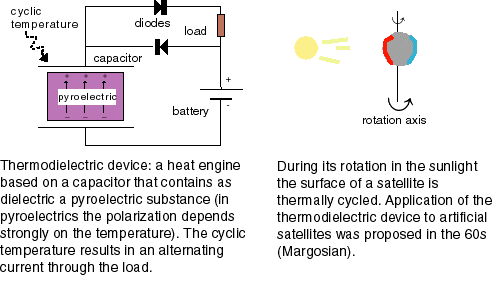
Margosian, PM (1965) Parametric study of a thermoelectrostatic generator for space applications. NASA techn. Note TN D-2763
Matsuno, K et al. (1999) Elongation of polypeptides in a simulated submarine hydrothermal system. Science 283, 831-833
Mitchell, P (1979) Keilin's respiratory chain concept and its chemiosmotic consequences. Science 206, 1148-1159
Nielsen, PE (1995) DNA analogues with nonphosphodiester backbones. Annu. Rev. Biophys. Mol. Struct. 24, 167-183
Shapiro, R (1988) Prebiotic ribose synthesis: a critical analysis. Origins of Life Evol. Biosph. 18, 71-85
VanHolde. KE (1980) The origin of life: a thermodynamic critique. In The origins of life and evolution, pp. 31-46 (eds. HO Halverson and KE VanHolde), Alan R. Liss, New York
VanWalraven, HS et al. (1997) The H+/ATP ratio of the ATP synthase from the cyanobacterium Synechococcus 6716 varies with growth temperature and light intensity. Biochim. Biophys. Acta 1318, 217-224
Woese, CR (1987) Bacterial evolution. Microbiol. Rev. 51, 221-271
Copyright © 1999 by Anthonie W.J. Muller
All Rights Reserved