Models with diffusion (Class1 models)
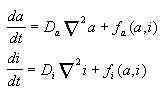
The first term in the right part represents the diffusion process, where
Da and Di
are diffusion constants for a and i correspondingly; the
second term represents other processes such as production and decay, they
may be written as
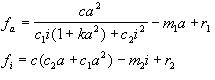
here if c1=0, then c2=1
and vice versa.
In this equations production is represented by the first and third terms,
second term represents the decay.
One of the most known and frequently used type of the systems of such a
kind can be derived if to set c1=1
(correspondingly c2=0), k=r1=r2=0,
namely
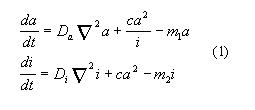
Resulting distribution may be monotonous or periodical and changes as a
function of parameters. The pattern formed inthis way may be stable or
oscillating with the time. The positioning of high concentrations is produced
by small internal or external asymmetries or by local disturbance.
This local high concentration can serve as signaling system, for instance
to initiate head formation in hydra. A pattern formed in this way has strong
self-regulatory properties.
Let us also to consider a special case of such a systems with feedback
loop.
To do this would require to set c2=0,
then
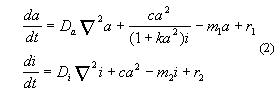
The small basic (activator-independent) activator production r1
can initiate the autocatalysis in areas of low activator concentration.
As we will see, this term is important if new centres have to arise during
growth or regeneration. In contrast, the basic inhibitor production can
suppress the appearance of secondary maxima, a feature which is important
if an ordered sequence of structures is to be specified by positional information.
If the activator production saturates at a high concentration due to term
1/(1+k*a*a), the activated area is self-regulated.
Other molecular realization of autocatalysis and lateral inhibition
principle may be, for instance, the processes in which inhibition effect
is realized by depletion of a substrate consumed in autocatalysis. From
mathematical point ofview this means that
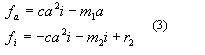
Also one has to remember here well-known in developmental biology Sel'kov's model:
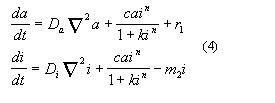
where n is known as Hill's number.
In this model one assumes that the activator reproduction is compensated
by selfregulated reproduction of the inhibitor. The stable pattern formation
is also possible in this case.