Below are extracts from my electronic article accepted for "CSTB Bulletin"
We considering as one of the most studied examples the network of genes-controllers of fruit fly segmentation. My consideration exploits following essential characters of gene networks. Each gene-member of the network encodes peptide-product which function is activation or repression of another gene (so called, targeting gene). These transcription factors recognise specific sequences of DNA in regulatory domains of the targeting genes and tightly bind to the targeting sites. Given member of the network produces transcription factors that specifically activate or repress of other netters, and vice versa. Complex network of autoregulatory and cross-regulatory actions functionally connects the genes forming thereby gene networks and cascades. Result of action of the network or cascade is peculiar spatial pattern of activity (expression) of the network members. Down-activated members, in turn, switch on structural genes in appropriate time and place. These genes, in turn, produce enzymes for following differentiation and morphogenesis of embryo rudiments. We preferably assume the concentration-dependent action of the peptide-regulator on the targeting gene. Namely, at low concentrations it acts as activator, while at high concentrations it acts as repressor (See Jackle et al., 1992).
Recruiting of new netters depends upon plasticity of recognition/action loops inside the network. Firstly, each transcription factor can recognize not unique DNA sequence (say, CATAAT), but family of similar sequences (say,
CCTAAT,
CATAAT, CATAAC, AATAAT, AATAAC ).Then, the sequence families for binding of different transcription factors can be very similar or overlap each other. In other words, the same target sequence can be recognized by two factors, especially if they have antagonistic action on the same targeting gene. These two characteristics are necessary and sufficient to ensure the recruiting phenomenon.
Consider following simple gene cascade having potential for complexification. Let us have exponential-in-distance gradient of morphogen M along longitudinal axis. Each genome initially consists of functionally coupled pair of gene regulatory elements (O-gene + A-gene).
![]()
Assume that M is site-specific transcription factor and its target is gene O. M has affinity to the CATAAT-like family of sequences and regulatory region of the O has two such sites. Assume that M acts on activity of the O in concentration-dependent manner: low concentrations of the M activate O, while high concentrations inhibit O. As a result, we obtain bell-shaped concentration profile of the O product along exponential-in-distance gradient of the M.
In its turn, O-product will activate A-gene in concentration-dependent manner also. Concentration profiles of the O- and A-products for wild species have following simple view:
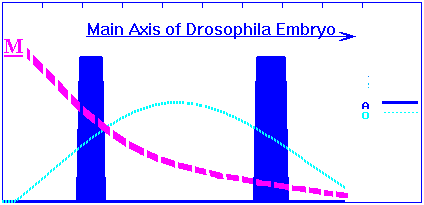
and corresponds embryo phenotype with two bands of the A-gene expression.
The wild-type genomes are two-string variables. Namely, the A-gene string is
CCTAATnCATAATnAATAATn,
where A, T, G, C is four-letter DNA code and n is spacer. The O-gene string has similar view. Each genome will test for "governing of development". This genotype-phenotype presentation achieving by "translation" of the strings into coupled ordinary differential eqs. as follows:
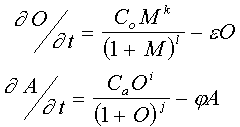
M is "maternal" morphogen. k and i are equal to the number of active targeting sites in the string (2 or 3 for the wild genes O and A, correspondingly), while l and j are equal to sum of predetermined affinities of the targeting sites.
Then assume, that two new genes O' and A' appear in the genome as a result of duplication of initial O + A pair:
![]()
(Features of appearance of new genes in evolution See Here).
Initial duplication of O+A gene pair followed by multiple point mutations in these O' and A' gene regulatory sites. Silent O' and A' extra-copies accumulate point mutations and there is possibility for shifting of target site specificity comparing with wild-type O and A pair. Only unique combinations of nucleotide substitutions in the silent pair of duplicates facilitate following recruiting of newly modified genes.
In discussing example, O-product recognize CATAAT sequence family. Hence, the change of the B-gene product recognition specificity to CAGAAT sequence would be appropriate for following evolution (that is B-product will bind to CAGAAT sequence). However, this must coincide also with shifting of C-gene product specificity to AGAAT sequence (that is C-product will bind to AGAAT sequence). "Waking up" of appropriate B + C pair is the case with very low but finite probability.
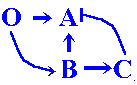
Hence, with time, appear the example of genome consisting from four genes, including proper to recruitment of new gene pair, B + C.
![]() The
B-gene must be the target for the O-product,
while the C-gene is the target for the B-product.
O-product activates B-gene in concentration-dependent
manner, and B-product acts on the C-gene by
the same manner.
The
B-gene must be the target for the O-product,
while the C-gene is the target for the B-product.
O-product activates B-gene in concentration-dependent
manner, and B-product acts on the C-gene by
the same manner.
As soon as in ![]() genome one point mutation change, say, second binding site in A
gene, we achieve the recruiting of the B + C pair:
genome one point mutation change, say, second binding site in A
gene, we achieve the recruiting of the B + C pair:
The mutant has following A-gene sequence: CCTAAT…CAGAAT…AATAAT (two "old" binding sites for the O-product and one site for B-binding overlapping with C-binding site). Hence, if one of the targeting sites mutates in such a way that it becomes target for concurrent binding of the B and C products, then this yields next equation for the A-activation:
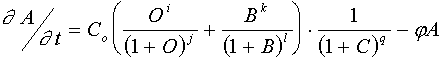
The recruiting of new genes via closing of new regulatory pathways really includes step-by-step "handing of steerage" from old gene-regulator to new one. Intermediate mutant genes regulated by both regulators is tight place for evolution of the net. Unique combination of kinetic parameters of gene activation will facilitate passing through "gate" to a new structure of the net.
Apparently the intermediate forms with doubly regulated A-gene have weaken fitness and will be eliminated by selection. However, if the mutant has selective advantages, say partial tolerance to the virus, then the "intermediates" will accumulate in population. By the way, this reminds well-known case of human crescent-cell anemia. Namely, bearers of the mutant hemoglobin gene suffer from anemia, but resistant to malaria. As a result, selection leads to spreading of the mutant gene among native inhabitants of Africa.
In future, the number of the new double mutant will grow and eventually complete mutant with absence of the O-binding sites.
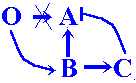
In our case it will have such gene A: CAGAAT…CAGAAT…CAGAAT.
The 4-gene cascades fulfill the morphogenesis successfully (the A-product concentration pattern satisfies the morphology of the wild type). This perspective monster shows new four bands of the C-expression.
Now main idea became clear: action of the activator B-product and the repressor C-product mimics action of the O.
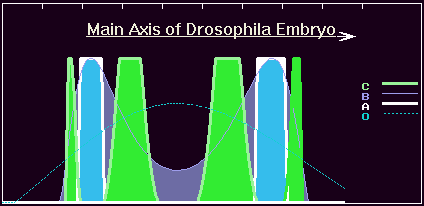
Namely, B-product gives two-wave gradient, similar to the A-concentration profile. The B-gradient activate A-gene by pure activation mechanism giving rise similar two-wave A-gradient. However, this pure activation mechanism gives too broad bands of the A-gene expression. Meanwhile the C-product is activated by the B in concentration-dependent manner, yielding four-wave C-concentration profile. It is essential that each pair of the C-product bands set boundaries for A-bands, narrowing them. Finally we obtain the same two-waive A-concentration profile, as in the case of the wild type genome.
Hence we achieve the recruitment of a pair of new members into simple cascade. As we can see, the same pattern of A-gene expression is achieved by action of more complicated gene network.
Apparently it is tutorial (kind of evolutionary game with strings), but it remains some essential features in organization of Drosophila segmentation network. Of course, such replacement of simple cascade by more complicated must be driven, indirectly or directly, by selection.
Complete paper see here
![]()
![]()
![]()
This page hosted by ![]() Get your own Free Homepage
Get your own Free Homepage