I.M.Sechenov Institute of Evolutionary Physiology and Biochemistry,
St.Petersburg, Russia
Diphenilhydantoin (DPH) produces nomalies in the cranio-facial region of animals and humans and disrupt cranio-facial and neural tube morphogenesis by inhibiting the arachidonic acid cascade. The pathogenesis is implicated for glucocorticoids and retinoids. The DPH is thought to interfere with the arachidonic acid cascade via the same nuclear receptor as for glucocorticoids (Kay et al., 1990). At the same time, DPH has similar chemical structure and teratogenic activity with phenobarbital (PB) and diazepam (DIA). In order to compare biologically active conformations of these three drugs with conformation of steroids and retinoids we have carried out computer conformational analysis of DPH, PB and DIA.
All the low energy equilibrium conformations of these three drugs were calculate with molecular mechanics method. Conformational analysis have been carried out with Allinger's program MM2 (Allinger et al., 1989). To compare spatial positions of functional groups in two compounds, they were superimposed in the common system of cartesian coordinates by means PC MODEL and ALCHEM packages.
The lowest-energy conformations of DPH and DIA are similar and elements of chemical structure supposedly essential for receptor interaction have approximately similar spatial position:
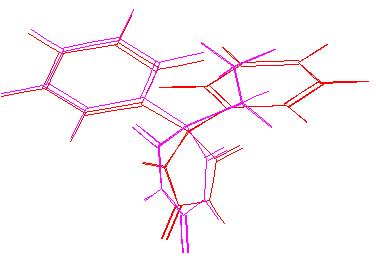 phenitoin&phenobarbital
phenitoin&phenobarbital
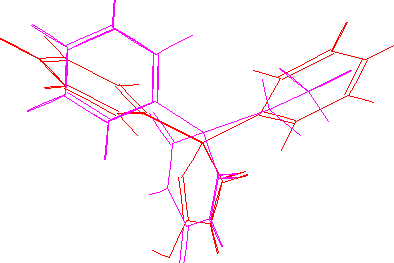 phenitoin&diazepam
phenitoin&diazepam
Comparison of steady low energy conformations of these drug molecules
with conformations of glucocorticoids and retinoid analogs ch 55 and
Am 80 reveals that the drugs under investigation hardly have nuclear
receptors identical with steroid or retinoid receptors.
LITERATURE
![]() *** this page is under development ***
*** this page is under development ***