We have a gap between 6.8min to 12.5min, which is
close to 100%(!), and everybody, who has experience with film
development knows that development results for the same film which vary
between the times
shown will be definitely NOT the same: either with 6.8min the same film
will be extremely underdeveloped or with 12.5 min (if the shorter time
is
OK) the film will be overdeveloped.
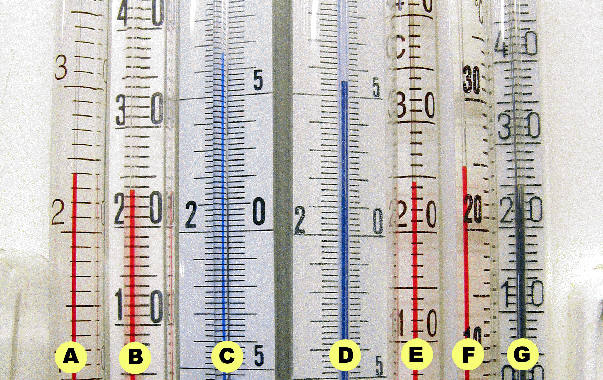
See the values, which have been determined by starting in some chilled
water. Temperature was raised by adding two batches of warm water:
Again we see poor uniformity! The last line shows the difference
between the lowest and the highest temperature. A shows 10% more than F
and G.
How can we live with such uncertainties? Easy! I've declared the
precision
mercury thermometer as the
standard thermometer,
and the
alcohol precision thermometer is my
work thermometer.
So the potential danger of the mercury is minimized (looks that vapours
are poisonous, and vapours may occur when the thermometer gets broken
), because this thermometer rests in a save wooden box in a save place.
I've glued a small table at an easy reachable location giving me the
read values of C and the 'real' values of D. As we can see from the
second table the
relative accuracy of both precision thermometers is nearly the same
(16.2
and 16.1), so they are "precise" in terms of relative accuracy, but
badly
adjusted!
Here is my way to achieve reproducible development conditions:
WORK THERMOMETER
Due to my table I know that when I want to have water of 19.2C
the scale must show 20,6C. If my work thermometer will get broken, I'll
buy a knew one and will standardize it with my 'standard' thermometer.
Give a thermometer filled with alcohol some time, at least 1 minute,
until you believe the temperature shown. Also take in account that
standard thermometers must be fully submerged to show exact
temperatures
(for me this means that the thermometer must be dipped so deep into the
liquid that the length of the alcohol column is under the liquid
level).
As a test: Have two glasses of water available, one filled with cold
tab
water, the other with warm tab water. Put the thermometer into the cold
water,
stir a little bit and wait until the temperature shown does not change
any
more. You will see, it will take some time. Now put the thermometer
into
the warm water. Temperature will raise, but see how slowly the upper
stable
value is reached, even when you stir the thermometer. It might take up
to
a minute until the temperature shown does not change any more. However:
When you see the
alcohol column reaching the upper limit of the
scale
immediately remove the thermometer from the warm water, otherwise
it
will burst! (In this case we don't have a common understanding what is
warm
and what is hot!)
A
table work thermometer -> standard temp looks like:
| Tread
from work thermo |
19.1C
|
20.1C
|
21.1C
|
22.1C
|
23.1C
|
equals Treal (accord. to
stand. thermo)
|
18.0C
|
19.0C
|
20.0C
|
21.0C
|
22.0C
|
(in this case T
read from work thermo is general
1.1C to high, but that's only due to the same linear increase). So when
I want to have 20C, I mix to read 21.1C! Luckily I just have to
add
or subtract a constant value.
In case we observe a linear behaviour of the deviation the table might
look different and we might have to plot a line.
See the following charts:
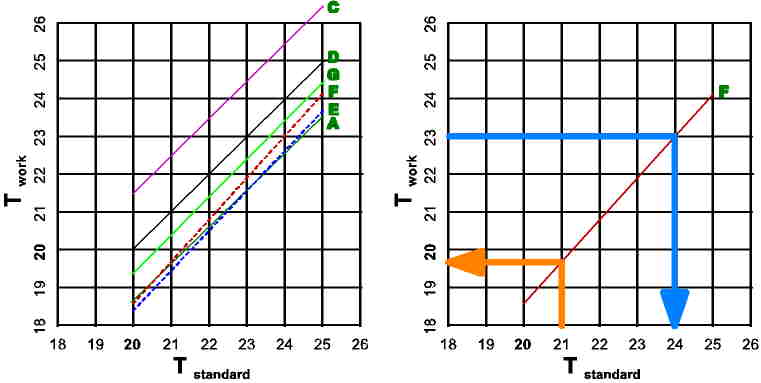
The left chart shows the temperature readable on each thermometer
(except
B) versus the temperature shown on the reference thermometer D. It is
clearly
visible that G, and C (and nearly A) have the same slope as D, so the
difference
is just a constant value for all temperatures. A, E, and F (the 'new'
way
to fabricate thermometers) show different slope, which make the
correction
of the value read more complex. This can be seen an the right chart:
When
we want to adjust the temperature of something to e.g. 21C, we have to
read
from the work thermometer
F19.7C. On the other side: when we
read
from this thermometer 23C it's in reality 24C.
STANDARDIZED DEVELOPMENT TIMES
My development times are adjusted in terms of
film/developer/dilution/agitation to get an optimum contrast in the
negatives for my enlarger and I measure temperature with my work
thermometer.
DEVELOPMENT AT
20C
Due to a very efficient heat insulated development tank (will be
described in future on my pages) start is always with 20C and end after
15min is with a maximum deviation of 0,5C from starting temperature.
These prerequisites allow to control the negative development process
completely and precisely.
And
electronic thermometers?
More accurate?
No,
not at all . Most electronic thermometers are specified to show
temperatures with +/- 1C and a resolution of 0,1K. We have here the
same as with the precision thermometers above: the
relative
accuracy is OK (resolution of 0,1K), while the
absolute
accuracy is not (+/- 1C)!
Why do we have the problems?
Temperature is a physical entity which we can't see (seeing is
our sharpest sense) but only feel, which is very imprecise. The
measurement is done by watching changes of physical properties which
react on temperature changes. With traditional thermometers it is the
thermal expansion of mercury or alcohol. The amount of liquid stored in
the bulb expands very constant proportional to temperature, and the
additional volume required is found in the capillary of the
thermometer. That's why the column grows with rising temperature, and
that's why a thermometer bursts when the
liquid column reaches the upper end. No space left for expansion
crackles
everything, because liquids and solids are not compressible (compared
with
gaseous substances) and thermal expansion can raise gigantic forces.
Now it is possible to produce very precise capillary tubes for
the column of the thermometer, but the volume for the bulb MUST exactly
match a given value otherwise the thermal expansion in absolute values
is too large or too small. If the required absolute value is not
exactly
hit, a volume too large will lead to 'nervous' instruments overreacting
to temperature changes, while the contrary is leading to 'lazy'
reactions
to environment changes. All this seems to be controllable! But as we
can see from the thermometer comparison above, it is difficult.
Now with electronic thermometers we look usually at resistance
changes of a material according to temperature, or there is something
like a law of nature that a voltage drops by -2mV/K of an internal
layer
of an integrated circuit (pn-area). This means that this device has to
handle a voltage difference of 0,2mV/0.1K , but 200µV is a very
small
voltage and difficult to handle. Again, the adjustment to
absolute values
is the problem, the
relative accuracy
is usually not the problem.
In general temperature is a physical property not very easy to
be measured very accurate. However, we can believe that we can measure
temperature quite reproducible for our own process. But when we tell
a friend to develop a film at this and that temperature with that
developer
(-dilution) and this agitation he very likely will fail to get
comparable
results, because his thermometer very likely will show different
temperatures
to ours.