|
Connective tissues are responsible for the form and shape of the animal body and, in addition, provide protection for vital organs and facilitate locomotion. The term connective tissue is also applied in a more restricted sense to structures such as dermis, tendons, fascia, bone, cartilage, and the capsules of the joint. All cells, however, make contacts with surrounding structures that involve connective tissues as components of the extracellular matrix. The matrix possesses chemical, physical, and mechanical properties uniquely suited to the function of tissues and organs of which the cells are a part. The extracellular matrix may be rigid (e.g., bone), elastic (e.g., blood vessel walls), compressible (e.g., cartilage), or liquid (e.g., synovial fluid). Most connective tissue matrices derive these properties by virtue of the content of fibrillar proteins, nonfibrillar macromolecules, and low molecular weight proteins and electrolytes. The properties of the matrix are therefore determined predominantly by the function of cells, specific for each tissue, which are responsible for synthesis of the matrix components. Many of the functions of the component cells, in turn, are influenced by the character of the extracellular matrix. The properties of connective tissues are also influenced by their relationships to the vascular system from which critical components are derived, such as water, electrolytes, and proteins. Indeed, the walls of blood vessels may themselves be considered as connective tissues. However, although some connective tissues are highly vascular (e.g., bone), others are essentially avascular (e.g., cartilage).
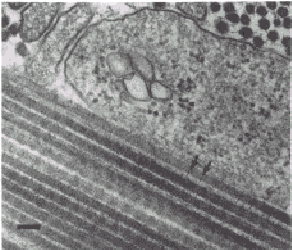
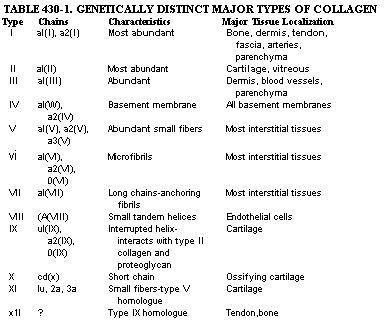
FIGURE 430-1. Electron micrograph of sections of 17-day-old chick Achilles tendon. Collagen fibrils are seen in longitudinal section in the lower left portion of the figure. These fibrils have a period of -67 nm, as indicated by the distance between the two arrows. Fibrils seen in cross-section at the upper portion of the figure have an average diameter of -50 run. Collagen fibers, which are made up of many fibrils, have diameters that range from 0.1 to 15 ~Lm. Bar = 100 run. (Courtesy of Dr. Romaine Bruns.) COMPOSITION OF EXTRACELLULAR MATRICES. The major components of the extracellular matrix are fibrillar proteins (collagens and elastin), globular proteins, complex carbohydrates, and, in the case of bone, the inorganic mineral phase. In most connective tissues the fibrillar proteins make up the bulk of the organic material. The elastic fibers consist of two distinct protein components. The most abundant is an amorphous protein (elastin) with no distinct periodicity by electron microscopy. The minor component associated with the elastin is a microfibrillar glycoprotein. Elastic fibers are most abundant in walls of large arteries and in some tendons and ligaments. In joints, however, collagens are the major fibrillar proteins. Collagens belong to a family of proteins that have similar chemical and structural properties. The term "collagen" is also used to refer to fibers or fiber bundles observed in tissue sections. The form of collagens in- tissues is determined by the type of molecule that predominates and by interactions with other components of the matrix. Collagen fibers usually have diameters of 0.1 ~Lm to 10 to 15 [Lm. The most abundant species, the interstitial collagens, such as those that predominate in tissues such as dermis, tendon, bone, and cartilage, have a characteristic banding pattern seen on electron microscopy with major periods of approximately 64 to 70 run (Fig. 430-1). Some collagens, such as those comprising basement membranes or others that are deposited pericellularly, appear amorphous and do not have a banded structure detected by electron microscopy. The collagen molecules of most collagens consist of three polypeptides (a chains) that have a characteristic and unique helical structure determined by the amino acid sequence. The collagen molecules of the interstitial collagens can be solubilized from some tissues. In solution these molecules behave as long, rigid rods with dimensions of approximately 300 x 1.5 nm. Each of the collagen polypeptide chains contains a glycine residue at every third position and is rich in amino acids such as alanine and proline. Collagens contain little phenylalanine and tyrosine and essentially no tryptophan, and, with the exception of type III collagen and the basement membrane collagens, usually lack cysteine in the body of the helical portion. Collagen chains in the course of synthesis also undergo unique post-translational modifications of several component amino acids. The most important of these modifications involves the introduction of a hydroxyl group in the 4 position of specific prolyl residues. The 4-hydroxyproline residues are considered to be responsible for stabilization of the collagen helix. In addition, there is a small amount of 3-hydroxyproline in most collagens whose function is not known. Specific lysyl residues also are modified by hydroxylation in the 5 position to form hydroxylysine. The hydroxyproline and hydroxylysine of collagens liberated by proteolytic cleavage of the polypeptide chains in the course of physiologic remodeling or pathologic degradation are not reutilized for collagen biosynthesis. Quantitation of the urinary excretion of these amino acids therefore provides some index of collagen turnover. Certain E-amino groups of lysines as well as hydroxylysines are oxidized to their respective aldehydes to form derivatives known as allysines and hydroxyallysines, respectively. Lysine, hyroxylysine, and their derivatives are involved in crosslinking between the chains that constitute the collagen molecules (intramolecular) as well as crosslinking one collagen molecule to another (intermolecular). In the case of the interstitial collagens the characteristic banding pattern is accounted for by an ordered staggered arrangement of the collagen molecules within the collagen fibrils and the collagen fibers. The manner of molecular packing within the fibril in turn is determined by the amino acid sequence. The way the molecules are staggered in the fibril, however, gives rise to regions in which the molecules overlap and others in which there is no overlap (Fig. 430-2). It is probable that the mineral phase of bone is deposited predominantly within the voids or holes of the nonoverlap region. The macromolecular structure of type IV collagen, the major fibrillar component of basement membranes, is very different from that of the interstitial collagens. Type IV collagen structure consists of a network of individual 390 nm-long molecules that are aggregated and crosslinked via identical ends to form a distinctive lattice. These proteins with structural homologies make up the collagen family. The different homologous species are referred to as types, with each type the product of a different (nonallelic) genetic locus. The 12 collagen types identified and characterized so far are listed in Table 430-1. Some tissues are characterized by a marked predominance of one typee.g., types II, IX, X, and XI in cartilage and type I collagen in bone. It is likely that each of these collagen types is largely responsible for the functional and morphologic properties of each connective tissue, although it has not yet been possible to relate function to a particular chemical modification.
FIGURE 430-2. A model of the packing of the collagen molecules within the collagen fibril. The molecules, each consisting of three helical polypeptide chains, are depicted as long rigid rods and are represented here by the cylinders. The length of the molecules is indicated by the long arrow. The structure gives rise to regions where adjacent molecules are in contact and others in which there are holes, indicated by the short arrow. It is suggested that the inorganic crystals of bone are located predominantly in these holes.
There is considerable information concerning the pathways of synthesis of various collagens. Even the genes from several animal species for the component polypepticle chains of several collagen types have been partially characterized, illustrating the enormous progress that has been made in the study of these molecules. Although the chains of the interstitial collagen molecules contain approximately 1000 amino acids, and the procollagen precursor contains additional polypeptide sequences at either end which would require a messenger RNA of approximately 4500 bases, the genes coding for each of the proal and proot2 chains have lengths of approximately 18,000 and 38,000 bases, respectively. The enormous size of the genes is due to the presence of approximately 50 intervening DNA sequences (introns), which do not code for amino acid sequences of the mature collagen chains. A possible sequence of events in the course of synthesis of the collagen molecule is shown in Table 430-2. Further complexities are illustrated by the finding that, in the case of type I collagen, the genes coding for the constituent chains are not even on the same chromosome. In humans, the gene for the al chain is on chromosome 17 and that for the a2 chain on chromosome 7. Despite the complexity of this synthetic process, there are several heritable disorders of connective tissue in which it is possible to demonstrate abnormalities in biosynthesis. Defects in synthesis of a particular type of collagen, type III collagen, have been demonstrated in a form of the Ehlers-Danlos syndrome (type IV), characterized by tissue friability and rupture of viscera and major blood vessels. Defects in hydroxylation of lysine have also been noted, which gives rise to another clinical form (type VI) of the Ehlers-Danlos syndrome. In addition, defects in processing of the procollagens have been demonstrated (Ehlers-Danlos syndrome type VII) as well as problems with crosslinking accounted for by failure to oxidize critical lysine and hydroxylysine residues (certain forms of cutis laxa). Insufficient synthesis of type I collagen has also been found in certain forms of osteogenesis imperfecta, particularly the classic dominant variety of moderate severity, associated with deafness and blue sclerae. In other cases of osteogenesis imperfecta, which fall into a different clinical and genetic grouping, additions or deletions of portions of the coding region for the procollagen extensions or the helical portions have been described. In one extraordinary case, no a2 chains are present in skin and bone nor are such chains secreted by fibroblasts. The defect lies in a portion of the extension peptides that does not permit normal assembly of the procollagen trimer. Thus, absence of a2 chains is not lethal; trimers of od chains can be formed.
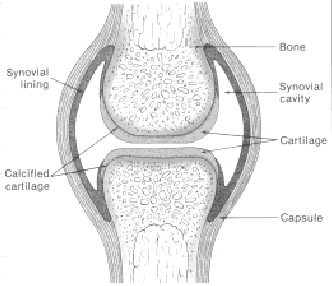
Other major components of connective tissues include the high molecular weight carbohydrates that make up the so-called ground substance of the interfibrillar matrix. These macromolecules, formerly known as mucopolysaccharides, are composed of a glycosaminoglycan portion (the complex carbohydrate itself) linked to a core protein. The core protein with the glycosaminoglycans attached is termed the proteoglycan subunit. In articular cartilage, proteoglycans constitute ap.prQxinVAely half the dry weight of the tissue. Another abundaht complex carbohydrate present in many tissues and the rhajor polysaccharide of synovial fluid is hyaluronic acid. The complex carbohydrates of cartilage are composed of high molecular weight polymers of the proteoglycan subunits, with the polysaccharide side chains of chondroitin sulfate and keratan sulfate linked to serine residues of the core protein. The polymeric components consist of these proteoglycan subunits bound to high molecular weight hyaluronic acid chains through interactions with another glycoprotein, called link protein. These proteoglycan aggregates are envisioned as occupying the spaces in cartilage surrounded by the collagen fibers and other components of the matrix. Both the proteoglycans and the collagens are synthesized by the articular chondrocytes. In general, the cells of the connective tissues interact with the complex carbohydrates and the collagen fibers through glycoproteins, which are probably specific for each type of connective tissue. For example, the cell membranes of epithelial cells interact with a glycoprotein known as laminin, which by interacting with type IV collagen, a unique heparan sulfate glycoprotein, and another protein, nidinogen, together make up the structure of the basement membrane. Many connective tissues and other cells interact with collagens through the protein fibronectin. Many cells have specific receptors for fibronectin which bind specifically to regions of this glycoprotein which contain the amino acid sequence Arg-Gly-Asp-Ser. Chondrocytes interact with their extracellular matrix through another glycoprotein called chondronectin. STRUCTURE AND FUNCTION OF JOINTS. The structure and characteristics of the diarthrodial joints are determined by the function of specific cells, which produce unique extracellular matrices. A typical joint such as that depicted schematically in Figure 430-3 has its characteristic components. The joint is ideally suited for the demands of weight bearing and motion, which must be operational with a minimum of wear over the lifetime of the individual. The functional properties of the joint are dependent upon a compressible, deformable cartilaginous surface that is properly lubricated and supported by relatively rigid subchondral bone. The stability of the joint, in turn, is determined by the connective tissue structure of the joint capsule, tendons, and ligaments and is influenced by function of muscles concerned with movement or support of that joint. The joint cavity is lined by a synovial membrane, which normally consists of one or two layers of cells. Some tendency to piling up of the cells is observed at the margins of the joint where the synovium is reflected.
The synovial lining cells are derived from connective tissue (they are not epithelial) and do not rest on a continuous basement membrane. In the normal synovium, at least two types of cells have been recognized. The type A cell is a phagocytic cell possibly related to macrophages; the type B cell is fibroblast-like. The joint cavity contains a characteristic synovial fluid. Its high viscosity is due to the presence of hyaluronic acid, which is probably synthesized by the synovial lining B cells. Water, electrolytes, and some low molecular weight serum proteins such as albumin are derived by filtration from the subsynovial capillaries. Glucose and electrolytes are present in normal al fluid at concentrations similar to those in plasma. In mation of the synovium, glucose entry is impaired and utilization increased to account for the lower synovial fluid glucose concentrations in some forms of arthritis, such as rheumatoid and septic arthritis. The concentration of proteins in normal synovial fluid is inversely proportional to their molecular weights. Albumin is therefore the most abundant protein. Plasma et,-macroglobutin, IgM, and fibrinogen are essentially excluded from normal fluids, possibly owing to molecular sieving effects of the hyaluronic acid in the interstitial regions of the synovium and -the 'syn&.,ial fluid. In joint inflammation, there ~s increased entry of the high molecular weight proteins into the synovial fluid. These pathologic fluids may thus form a fibrin clot, whereas normal fluids do not. This is to be distinguished from the so-called mucin clot, which is composed of a protein-hyaluronic acid complex that can be produced by the addition of dilute acetic acid to synovial fluid. A tight, ropy mucin clot is characteristic of normal or traumatic fluids, whereas inflammatory fluids tend to produce a fragmented clot or a dispersed sediment upon addition of acetic acid. The clinical diagnostic usefulness of the mucin clot test is not uniformly accepted, however. The cartilage of the diarthrodial joint is avascular, and the chondrocytes must receive their nourishment from the synovial fluid; products of their metabolism are in turn disposed of through the synovial fluid. The function of the articular cartilage is critically dependent upon the interaction of the fibrillar collagenous matrix and the proteoglycans. By virtue of the highly negative charge on the proteoglycans, these molecules occupy a large domain. The extent to which articular cartilage is deformed on compression and the ability of the cartilage to regain its shape following release from compression is determined by interactions of the proteoglycan aggregates with the fibrillar matrix. It is envisioned that alternate compression and relaxation of the cartilage during motion and weight bearing are responsible for movement of fluid and electrolytes in and out of the cartilage interstitiurn and provide a mechanism for the nutrition of the chondrocytes. The properties of articular cartilage with its surface in contact with synovial fluid account for the extraordinarily low coefficient of friction upon movement of the joint. The viscous hyaluronic acid that serves a lubricating function for the synovial membrane is probably not responsible for lubrication of the articular cartilage itself. Other components such as lubricating glycoproteins interact with components on the surface of the articular cartilage, providing a so-called boundary type of lubrication. The very fact that the cartilage is capable of deforming under load and regaining its shape following release of the load also contributes to the low coefficient of friction of moving joints. This elastic, spongy nature of cartilage allows it to weep fluid when squeezed under high loads, which, in part, creates a film of lubrication at the head of the moving surfaces. It is also postulated that the highly ordered structure of the cancellous bone supporting the articular cartilage dissipates the shock of impact under loading and permits some of the mechanical forces to be dispersed away from the articular cartilage. ALTERATION OF STRUCTURE AND FUNCTION OF JOINTS IN DISEASE. When joints are subjected to mechanical or chemical trauma, there are several predictable responses that occur in the articular cartilage and surrounding structures. Repeated mechanical trauma is associated with loss or decrease of the proteoglycan of the cartilage matrix, which can be appreciated histologically as a loss of the staining properties (e.g., metachromasia) attributed to this component. The mechanism for the proteoglycan loss probably involves the secretion of proteolytic enzymes, by the chondrocytes or by cells in the synovial fluid or the synovial lining, which, at neutral pH, cleave the core protein of the proteoglycans near the linkage region with the hyaluronic acid. Following cleavage of the core protein, the partially degraded proteoglycan is leached from the matrix. Alterations of this type have been observed following arthrotomy or bleeding into the joint. Since the articular chondrocytes have the capacity to resynthesize proteoglycans, if the mechanical injury is only temporary, some structure of the matrix can be restored. However, persistent deficiency of cartilage proteoglycan is accompanied by alteration in mechanical properties of the matrix manifested by an increased tendency of the cartilage to deform under load and a decreased ability to regain form following removal of the load. Only late in the course of injury is the collagenous component of the matrix affected. Although articular chondrocytes have retained the capacity to replicate and to increase, often in clusters, in response to chronic injury, these cells have a limited capacity to resynthesize type II collagen and reconstitute the normal fibrillar matrix. The proliferation of cartilage cells in response to trauma and mechanical stress may also be followed by vascularization and activation of the enclochondral sequence, resulting in formation of osteophytes, usually at the margin of joints. Trauma may also produce reactions in the synovium, probably mediated by altered vascularization in addition to increased numbers and activity of the synovial lining cells. These synovial reactions may in turn result in the formation of increased synovial fluid, manifested clinically as effusions. The composition of these synovial fluids closely resembles that of normal synovial fluid with respect to viscosity (the concentration of hyaluronic acid) and the type and relative concentration of the protein components (predominance of albumin and absence of high molecular weight plasma proteins such as fibrinogen, IgM, and ot,-macroglobulin). Thus, a mild degree of "synovitis" may be a component of either acute or chronic injury. Under these circumstances, however, few cells (100 to 500 per cubic millimeter) are present in the synovial fluid; lymphocytes and monocytes predominate, whereas polymorphonuclear leukocytes are scarce. In almost all types of joint inflammation, however, whether acute, as in urate gout or pseudogout (calcium pyrophosphate deposition disease), or chronic, as in typical rheumatoid arthritis, there is an exudation of cells, particularly polymorphonuclear leukocytes, into the synovial cavity. In these inflammatory joint diseases, the synovial fluid usually is characterized by a decreased viscosity, an inability to form a normal tight mucin clot, and an increased concentration relative to normal of macromolecules such as immunoglobulin, a,-macroglobulin, and fibrinogen. Enzymes released from the inflammatory cells have the capacity to degrade the proteoglycan core protein and collagen of the articular cartilage and surrounding structures if their concentrations (activities) exceed those of inhibitors present in synovial fluid and synovial tissues. The synovitis seen, for example, in rheumatoid arthritis may be particularly intense with chronic inflammatory cells present, especially at the margins of the joint where the synovial membrane is reflected. With persistent synovial inflammation, a mass of proliferating cells (pannus) may burrow beneath the articular cartilage and subchondral bone or appear to work its way over the surface of the articular cartilage, degrading matrix structures in its wake. These degradative processes are probably mediated by metalloproteinases, which can specifically degrade components of the extracellular matrix such as native collagen (collagenase), denatured collagen (gelatinase), and the core protein of the proteoglycans (stromelysin). Serine proteinases also play a role in this process. These degradative enzymes are produced not only by inflammatory cells but also by mesenchyrnal cells such as synovial fibroblasts and articular chondrocytes. The activity of the latter cells is markedly influenced by inflammatory cytokines such as interleukin 1 and tumor necrosis factor. Alterations also occur in the subchondral bone in joint disease. In the noninflammatory forms, the subchondral bone may increase in mass by new bone formation (sclerosis). In contrast, in inflammatory joint disease, subchondral bone is frequently resorbed, producing the typical radiologic appearance of juxta-articular osteoporosis. Diaphyseal cortical bone is usually not thinned until late in rheumatoid disease; in some subjects, it may even be increased in thickness owing to periosteal new bone formation. When the inflammation subsides, bony erosions may heal, and some restoration of the diffuse juxta-articular bone loss may also occur. Defects or clefts in articular cartilage, on the other hand, generally do not heal with restoration of the original form because of the limited capacity of chondrocytes to resynthesize the specific collagenous fibrillar component of extracellular matrix. Each of the extracellular components of the matrix of the joint structures has its unique pattern of composition with respect to the collagen type, proteoglycan, and glycoprotein component. The composition of these matrices must in turn determine their function. Return of function with healing of disease therefore requires restoration of the original composition, which, in turn, is dependent upon the ability of the tissue to undergo remodeling. The limited capacity for remodeling of some tissues, such as articular cartilage, therefore accounts for disability in several of the rheumatic diseases. Thus, in instances in which structure is sufficiently distorted and return of function impossible, the only alternative may be to use the artificial surfaces of joint prostheses to permit or regain motion and weight-bearing capacity and to alleviate pain. |
ENTRANCE | HOME | 1 | 2 | 3 | 4 | LINKS | FUN STUFF | BULLETIN BOARD | BOOK STORE | DISEASES | SEARCH