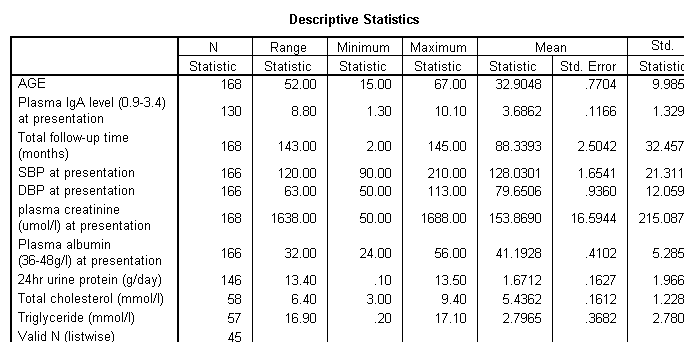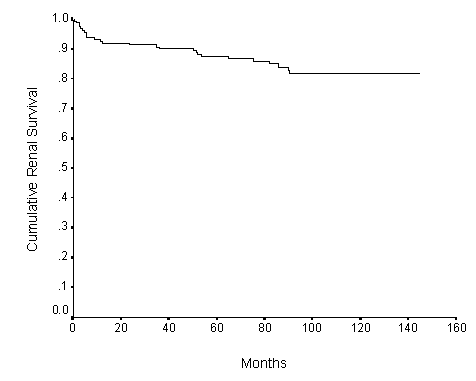

¡@
Ten-Years Follow-up of 168 Chinese Patients With IgA Nephropathy In Hong Kong
Dr Kelvin Ho
¡@
INTRODUCTION
IgA nephropathy was first reported by Berger and Hinglais in 1968.1 Since then investigators from various parts of the world have reported their local experience. It is the most common primary glomerulonephritis in the world 2 and accounted for 35% of all primary glomerulonephritis in Hong Kong.3
IgA nephropathy has divert clinical presentations ranging from asymptomatic proteinuria and/or microscopic haematuria to advanced renal impairment. It is not a benign disease. Up to 30% of patients with IgA nephropathy progress to end stage renal failure.4,5,6,7 There is an increased prevalence of hypertension in patients with IgA nephropathy. Hypertension is probably the most important of the various clinical factors suggested to be prognostic indicators of renal impairment. Ho et al has reported an increased incidence of hypertension in patients with IgA nephropathy and in their first-degree relatives. A positive family history of hypertension has also been suggested for the first time to be an independent risk factor of renal impairment.8 Various pathohistologic findings of renal biopsy have been used to predict the long-term outcome of renal survival.4,5,6,9-14 Some scoring systems based on pathohistologic findings have been reported. However, the interrelationship between renal pathology and clinical characteristics of patients were not clearly defined.
The aim of the current study is to review the clinical characteristics of Chinese patients with IgA nephropathy in Hong Kong. We identify the clinical factors and renal pathologies of patients at presentation as risk factors of renal impairment. We also look at the interrelationship between the clinical risk factors and renal pathohistologies.
¡@
PATIENTS AND METHODS
Patients with the diagnosis of primary IgA nephropathy based on renal biopsy at the Prince of Wales Hospital from July 1980 to June 1991 were recruited into the study. The medical records of these patients were reviewed. Patients_ clinical characteristics at the time of renal biopsy were analyzed. It included age, sex, presence or absence of hypertension (defined as blood pressure greater than 140/95 mm Hg or taking antihypertensive medications), presence or absence of hypertension in the first-degree relatives of patients (defined as blood pressure greater than 140/95 mm Hg or taking antihypertensive medications), 24 hours urinary protein, plasma creatinine, hepatitis B surface antigen (HBsAg), IgA immunoglobulin, and lipid levels. Patients with systemic diseases including systemic lupus erythematosus, Henoch-Schonlein purpura, chronic liver diseases, alcoholism and abnormal liver function tests were excluded from the study.
The indications of renal biopsy included microscopic hematuria; repeated proteinuria of more than 0.5gm/day; the presence of renal impairment with relatively normal size kidneys; and young hypertensive patients with non-renal causes excluded. Percutaneous renal biopsies were obtained by using Tru-cut or Temno (Bauer Medical International, S.A.) biopsy needles. Specimens containing less than 10 glomeruli were excluded from the study. The biopsy specimens were processed for light microscopy, immunofluorescence studies, and electron microscopy studies.15 IgA nephropathy was diagnosed by the presence of predominant IgA deposits in the mesangium and occasionally along the peripheral capillary basement membrane in a granular pattern.
A semiquantitative analysis of glomerular, tubular and interstitial damages in the renal biopsies were examined under light microscopy. The pathologic changes were classified independently into three grades 10,16 according to a modified classification used by Lee et al.17 Grade 1 denoted the presence of minor glomerular abnormality with mild segmental mesangial proliferation and less than 10% tubulo-interstitial involvement. Grade 2 indicated the presence of moderate degree of mesangial proliferation, occasional areas of glomerulosclerosis and crescent formation (in less than 20% of the glomeruli) and the tubulo-interstitial involvement of less than 30%. Grade 3 denoted the presence of the above but with more than 30% glomerulosclerosis and tubulo-interstitial changes.
¡@
STATISTICAL ANALYSES
All numerical data are expressed as mean+/-standard deviation. The difference of means between groups were tested for significance by one-way analysis of variance (ANOVA). Spearman_s rank test was used for correlation analysis. The renal survival from the date of renal biopsy to different end points including renal failure requiring dialysis or transplant, and, death was estimated by Kaplan-Meier method. The influence of prognostic factors on the renal survival curves was analyzed with univariate analysis by log-rank test and multivariate analysis by Cox regression.
¡@
RESULTS
One hundred and sixty-eight patients diagnosed to have primary IgA nephropathy were recruited into the study. Twenty-four patients required long term dialysis. Nine patients received cadaveric renal transplant. Four patients died over various periods of time after the diagnosis. One patient died of cerebral vascular accident, 1 of septicemia, and 2 of general condition deterioration.
The mean age of all patients at biopsy was 32.9+/-10 years (S.D.).The youngest was 15 years and oldest was 67 years. The age distribution is shown in Table 1. The highest incidence (47.6%) of all cases occurred in the age group between 25 to 34 years. The male to female ratio is 1:1.5 with 66 male and 102 female patients.
The hypertension status of patients and their family members are shown in Table 2. Forty-eight of 168 patients had hypertension at presentation. Only 137 of the 168 patients had data of their families_ information of hypertension available for analysis. Forty-seven (34.3%) of these 137 patients had one or more of their first-degree relatives were hypertensive. Thirty-nine of these 47 cases (83%) with positive family history of hypertension are due to hypertension in one or both of their parents. Only 8 (17%) are due to hypertension in their siblings alone.
Incidental finding of asymptomatic proteinuria and hematuria is the commonest presentation. Other common clinical presentations of the condition are shown in Table 3. There were 66 patients (39%) with plasma IgA immunoglobulin concentration greater than normal range (>3.4g/l). Nineteen of 126 (15.1%) patients screened for hepatitis B virus were found to be carrier. Hypocomplementemia was not found.
One hundred and sixty-seven biopsies were available for pathologic grading from 1 to 3. One renal biopsy was excluded for grading due to inadequate glomeruli in the biopsy. The results of the renal biopsy grading at presentation and the eventual outcome of patients are shown in Table 4. Sixteen of 24 patients required dialysis had grade 3 renal biopsy, 6 had grade 2, and 2 had grade 1. All 4 patients died had renal biopsy of grade 3. Forty-three (16/37) and ten (6/59) percents of patients with grades 3 and 2 progressed to end stage renal failure requiring dialysis. The various clinical characteristics of patients in the 3 pathologic grades are shown in Table 5. There is no significant difference in age, sex, plasma IgA and triglyceride levels of patients between different grades. There are significant differences in the plasma creatinine, albumin, total cholesterol, and urinary protein at presentation between patients of the 3 grades. Higher pathologic grades are associated with higher levels of plasma creatinine, total cholesterol, urinary protein, lower plasma albumin. Hypertension, positive family history of hypertension, plasma creatinine, albumin, total cholesterol, and urinary protein correlated significantly with the pathologic grading of biopsy (Spearman p<0.004).
The cumulative renal survival rate was 92% at 1 year, 87.5% at 5 years and 81.8% at 10 years. The Kaplan-Meier survival curve is shown in Figure 1. Hypertension (Figure 2), positive family history of hypertension (Figure 3), renal impairment (plasma creatinine >120umol/l) (Figure 4), total cholesterol, proteinuria >1g/day, and pathologic grades of biopsy at presentation (Figure 5) correlated significantly with renal survival by univariate analysis (p < 0.05). Hypertension, family history of hypertension, renal impairment and pathologic grading of biopsy are independent risk factors of renal survival when analyzed by multivariate analysis with Cox regression (p<0.05).
¡@
DISCUSSION
One hundred and sixty-eight patients with IgA nephropathy followed-up over 10 years in our hospital were analyzed in the current study. It enables us to study the clinical characteristics as prognostic factors and long-term natural history of the disease. Some of the clinical and histopathologic characteristics of patients with IgA nephropathy in the Chinese population of Hong Kong have been reported by us previously.10,18
IgA nephropathy commonly manifests in young adults and the incidence peaks at age between 25 to 34 years. The disease is occasionally diagnosed in older patients when renal biopsies are done for investigations of increasing proteinuria and/or renal impairment. The incidence of the disease is higher in Asian populations.18,19,20 The different policy of less reluctant to perform renal biopsy in Asian countries for the investigation of asymptomatic proteinuria and/or microscopic hematuria is a possible explanation. The current study shows a slightly higher female to male ratio, which is close to Japan, Singapore, and our previous reports.18,19,20 The cause of a different sex ratio to Caucasians is unclear. Genetic and environmental factor is a possible explanation. The incidence of hepatitis B surface antigen carrier in patients with IgA nephropathy is 15.1%, which is higher than 9.5% in the general population of Hong Kong. 21 Hepatitis B virus antigenemia has been suggested to play a pathogenic role in this subset of patients with IgA nephropathy.
The genuine duration of the disease from the onset to diagnosis by renal biopsy remains difficult to determine due to the nature of insidious onset of the disease. The incidental finding of proteinuria and/or hematuria remains the most common presentation. IgA nephropathy is not a benign disease. The incidence of mortality and chronic renal failure requiring dialysis rises with longer follow-up. There were 4 deaths and 24 patients required long term renal replacement therapy in our group of patients. The overall cumulative renal survival of our patients was 87.5% at 5 years and 81.8% at 10 years. Our result is comparable to other large cohort studies in different parts of the world.6,12,13,22
Our group found a higher prevalence of hypertension 28.6% in patients with IgA nephropathy compared with 17% in men and 5% in women in the Chinese population of Hong Kong.23 Similar finding of increased incidence of hypertension in patients with IgA nephropathy is also found in patients of Australia, the United Kingdom, and various parts of the World. 5,6,8,24,25 We also found increased incidence of hypertension 34.3% in the first-degree relatives of patients supporting the suggestion of a genetic predisposition of hypertension in patients with IgA nephropathy. 26,27 The association of IgA nephropathy and familial hypertension is futher suggested by an indirect evidence of finding an abnormal sodium-lithium countertransport kinetic parameter (Vmax/km ratio) in patients with IgA nephropathy and their first-degree relatives with hypertension as shown by Ho et al.8,28 Patients with IgA nephropathy are at increased risk of progressive renal failure when they have hypertension, and their first-degree relatives with hypertension.
The pathologic grading system of renal biopsy in our study is not affected by age and sex of patients. It correlates well with the various clinical factors reflecting the severity of renal impairment. Higher grades of biopsy are associated with higher plasma creatinine, urinary protein, and lower albumin. These are independent risk factors of renal impairment found in the current study and in previous studies by other investigators. The pathologic grading system also correlates significantly with the renal survival. This grading system is comparatively easy to use and reproducible which makes it an ideal clinical tool as an independent prognostic indicator.
Delay in diagnosis and treatment due to late presentation has been suggested to be a major cause of higher renal failure rate in these patients.14 It is a possible explanation since the renal survival rate is not much better in the Asian patients who had earlier diagnosis by renal biopsy. From our study patients with low grade pathologic damage were more likely remaining to have stable renal function. It was those who had moderate to advance (grade 2 and 3) renal damage at presentation progressed to renal failure despite close monitoring and treatment. Detection of the disease at an early stage by unrinalysis screening and to start treatment in high risk patients to prevent progression of renal damage may be able to reduce renal failure in IgA nephropathy. Identification of high risk patients by the above clinical indicators and pathologic grading are most important. There is no specific treatment for IgA nephropathy although fish oil has been suggested to be useful in retarding the rate of renal function deterioration.29 Tight control of blood pressure remains the most important treatment for the prevention of progressive renal impairment.
In conclusion, IgA nephropathy is a heterogeneous disease with many different risk factors. Patients with hypertension and positive family history of hypertension are at increased risk of renal impairment. The importance of a positive family history was undermined previously. Early diagnosis and identification of high risk patients are most important. Regular follow-up and well control of hypertension remains the most important management for the prevention of progression to renal impairment. Our pathologic scoring system of renal biopsy is a simple but informative scoring system, which enables us to identify the subgroup of patients at highest risk of progressive renal impairment to plan for long term management at an early stage.
¡@
REFERENCES
¡@
Table 1: The age distribution of 168 patients with IgA nephropathy
|
Age range (years) |
Number (%) |
|
15-24 |
31(18.5) |
|
25-34 |
80(47.6) |
|
35-44 |
35(20.8) |
|
45-54 |
17(10.1) |
|
55-67 |
5(3) |
¡@
¡@
Table 2: Status of hypertension in patients with IgA nephropathy and their first degree relatives. *137 cases had family history of hypertension available for analysis.
|
Hypertension in |
Yes(%) |
No(%) |
Total number |
|
Patients |
48(28.6) |
120(71.4) |
168 |
|
First Degree Relatives* Parents Siblings |
47(34.3) 39(83) 8(17) |
90(65.7) |
137 |
¡@
¡@
Table 3: The clinical presentation of patients with IgA nephropathy
|
Presentation |
Number (%) |
|
Asymptomatic proteinuria |
39(23.2) |
|
Microscopic hematuria |
23(13.7) |
|
Asymptomatic proteinuria and hematuria |
53(31.5) |
|
Macroscopic hematuria |
32(19) |
|
Nephrotic syndrome |
8(4.8) |
|
Nephritic syndrome |
2(1.2) |
|
Advanced renal failure* |
11(6.5) |
*
=plasma creatinine > 500umol/l.¡@
Table 4: The three renal biopsy grading at presentation and the length of follow-up.
|
Grade 1 |
Grade 2 |
Grade 3 |
Total |
|
|
Number(%) |
71(42.5) |
59(35.3) |
37(22.2) |
167(100) |
|
ESRF |
2(2.8) |
6(10) |
16(43.2) |
24 |
|
Death |
0 |
0 |
3 |
3 |
|
Follow-up in months (mean+/-S.E.) |
88.5+/-4.2 |
94.4+/-3.4 |
79.6+/-5.5 |
¡@ |
ESRF: end stage renal failure. ( ) is the percentage of patient within grade.
¡@
Table 5: The clinical characteristics of patients divided into 3 renal biopsy grades where data are in mean+/-S.D.
|
Renal Biopsy |
Grade 1 |
Grade 2 |
Grade 3 |
r (p) |
|
Age(years) |
31.7+/-10.3 |
33+/-10.3 |
34.9+/-8.7 |
N.S. |
|
Male/Female |
25/46 |
25/34 |
16/21 |
N.S. |
|
Hypertension(yes/no) |
11/60 |
16/43 |
20/17 |
0.33 (0.000) |
|
Family History of Hypertension(yes/no) |
12/51 |
17/32 |
18/7 |
0.39 (0.000) |
|
IgA Level(g/l) |
3.7+/-1.2 |
4.0+/-1.6 |
3.1+/-0.9 |
N.S. |
|
Creatinine(umol/l) |
91.2+/-59.5 |
96.6+/-33.6 |
360+/-379.4* |
0.44 (0.000) |
|
24hup(g/day) |
1.2+/-2.2 |
1.6+/-1.7 |
2.7+/-1.6~ |
0.27 (0.001) |
|
Albumin(g/l) |
42.4+/-5.1 |
41.3+/-4 |
38.8+/-6.5# |
-0.26 (0.001) |
|
Cholesterol(mmol/l) |
4.9+/-1.1** |
5.5+/-1.3 |
6.1+/-1.0 |
0.37 (0.004) |
|
Triglyceride(mmol/l) |
1.9+/-1.6 |
3.3+/-3.3 |
3.4+/-2.9 |
N.S. |
g/l = gram/liter.
r = correlation coefficient.
p = significance (Pearson).
N.S. = not significant.
24hup(g/day) = 24 hours urinary protein (gram/day).
*
p<0.001 when grade 3 compares with grades 1 or 2.~
p= 0.002 and 0.001 when grade 3 compares with grades 2 and 1 respectively.#
p=0.02 when grade 3 compares with grades 1 or 2.**
p=0.004 and 0.07 when grade 1 compares with grade 3 and grade 2 respectively.¡@
¡@
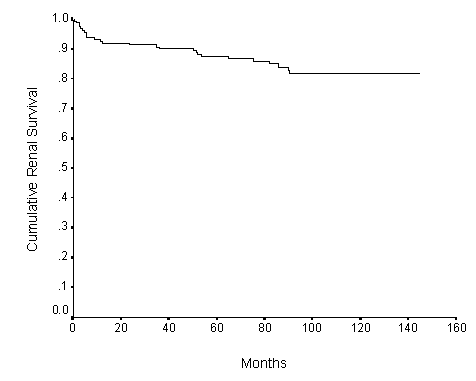
Fig.1. The cumulative renal survival of patients with IgA nephropathy over 10 years after biopsy (n = 168). n = patient number.
¡@
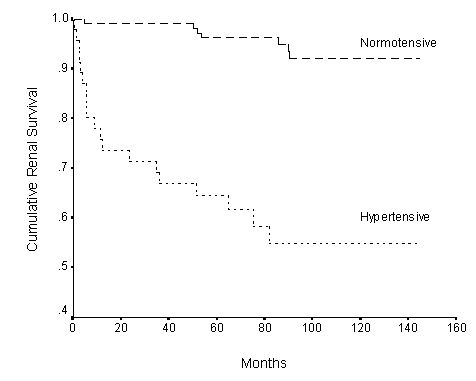
Fig.2. Renal survival curves for hypertensive (n=48) and normotensive patients (n=120). The significance of correlation P=0.009. n = patient number.
¡@
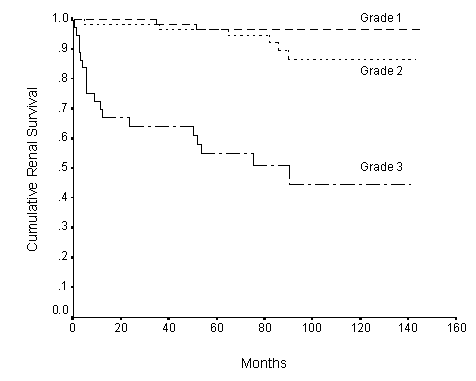
Fig.5. Survival curves of renal biopsy histologic grading at presentation. Number of patients in Grade 1 =71, Grade 2 = 59, Grade 3 = 37. The histologic grading is significantly associated with renal survival. P=0.0004.
¡@
¡@
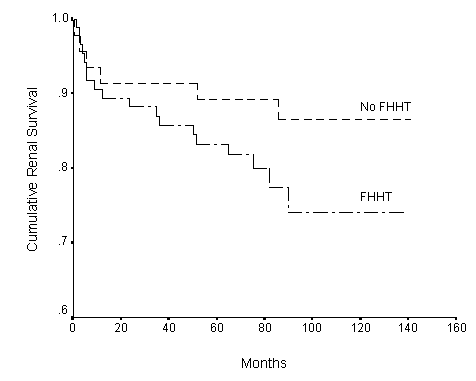
Fig.3. Renal survival curves for patients with (FHHT) (n=47) and without (No FHHT) (n=90) family history of hypertension (p<0.001). n = patient number.
¡@
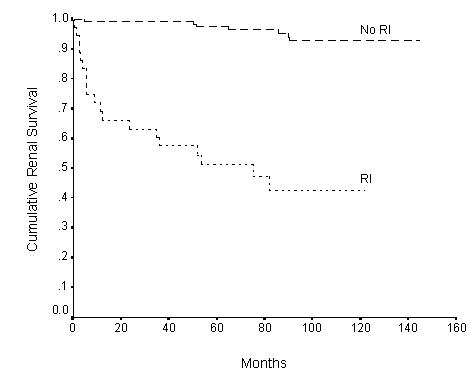
Fig.4. Renal survival curves in patients with (IR) (n=37) and without (No IR) (n=131) renal impairment (creatinine >120umol/l) at presentation. P=0.04. n = patient number.
¡@