Weekly Outlines February to March
Latest Update March 12 2005
Course notes may be found by

 Opening Day February 17
Opening Day February 17
- Introduction of course topics
- Periodic table; columns, rows families, shells
- Remember the three simple rules you were given:
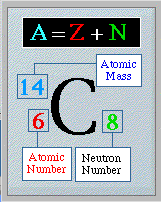
- The period in whcih the atom is found on the periodic table is the number of shells in that particular atom.
- Outer shell can achieve a maximum of 8 electrons; no exceptions.
- Inner shells will never exceed 18 electrons; this will start to occur when the atomic number is greater than 56 or 57. (Not a worry at this level)
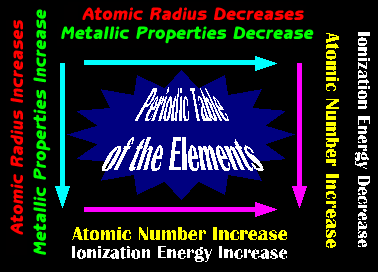
- Introduction to the Periodic Table;
 A discussion on the catagorization of the periodic table was presented.
A discussion on the catagorization of the periodic table was presented.

- Introduction to chemical families:
- Halogens
- Noble gases
- Alkali metals
- Alkaline earth metals
- Coinage metals
- Representaive elements, transition metals and the two series, lanthanide (rare earth elements) & actinide series.
- States of matter
- What's on the Internet; a quick outline.
- Physical & Chemical changes
- Two videos: i) Physical and Chemical Changes ii) Atoms & Their Electrons
 Week Two Feb 21 & 24
Week Two Feb 21 & 24
 Week Three Feb 28 to March 4
Week Three Feb 28 to March 4
- Use of Electronegativity to help predict bond type.
- Discussion of ionic and covalent bonding using Lewis dot diagrams
Here's a 3-D graph of electonegativities

- Balancing equations, with a worksheet. Make sure you ask questions if problems arise.
- Experimental evidence of nonpolar and polar molecules
- Pure covalent, polar covalent and ionic: the bonding continuium.
Test on everything Monday (A touch of nomenclature is always included).
- Five main types of chemical reactions: on which you should know examples of each. Try to remember the Lego examples.
- synthesis
- decomposition
- single replacement or displacement
- double replacement or displacement (special category = neutralization)
- combustion, complete and incomplete
- Chemical nomenclature, formula and balancing equations will be done on an ongoing activity. See page 97 and 98 for charts of ions and polyatomic ions.
or Click Here
for my chart.
- Introduction to the mole: Skittle example.
- Intoduction to formula weights, example: What is the formula of iron (III) sulfate Fe2(SO4)3?
atomic weight of iron X 2 + atomic weight of sulfur X 3 + atomic weight of oxygen X 12
- Internet assignment C due on Monday March 7. Late assignments will NOT be accepted.
Internet assignment D (Triton Project) due Thursday March 10
- Isotopes ---> Know what are they and examples of isotopes.
Working with relative abundances; be able to solve both ways: Determining atomic weight from given relative abundances and
If given atomic weights of isotope and sample determine relative abundances.
Continue on to page two

 Week Four Mar 7 to 10
Week Four Mar 7 to 10

 Opening Day February 17
Opening Day February 17



 Week Two Feb 21 & 24
Week Two Feb 21 & 24



 Week Three Feb 28 to March 4
Week Three Feb 28 to March 4


 Week Four Mar 7 to 10
Week Four Mar 7 to 10