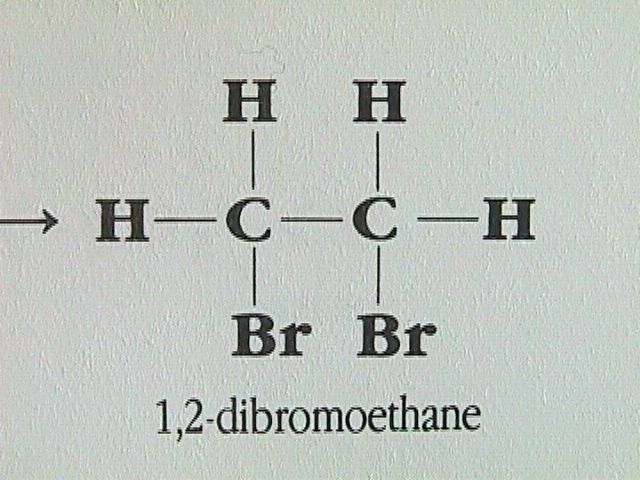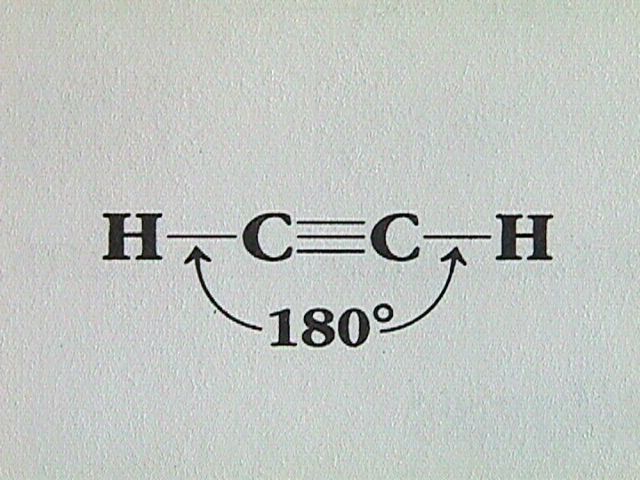Organic Chemistry
Characteristics of Organic Compounds:
1. Organic compounds contain covalent bonds.
Organic chemistry is dominated by covalent bonding.
2. Organic compounds contain carbon atoms joined together in chains or rings.
The ability of carbon atoms to bond together and form long chains or rings of atoms is called catenation. Carbon, more than any other element, can form these extended chains and rings.
3. Dispersion (London) forces are the principal intermolecular forces acting between organic molecules.
4. One molecular formula can often represent a large member of different organic compounds.
Different compounds with the same molecular formula are called isomers.
5. The properties of organic compounds are determined by the presence of certain groups of atoms within the compound.
Groups of atoms that impart specific properties to an organic compound are called functional groups.
Hydrocarbons:
Organic compounds containing only carbon and hydrogen are called hydrocarbons. The simplest hydrocarbon has the formula CH4.
Hydrocarbons can be subdivided into two main categories, aliphatic (insoluble in water and less dense than water, like fats) and aromatic.
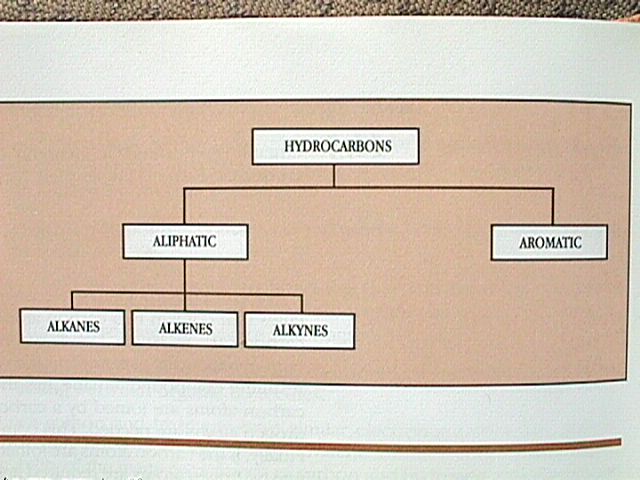
The aliphatic compounds are subdivided into alkanes, alkenes, and alkynes.
Alkanes contain only carbon carbon single bonds.
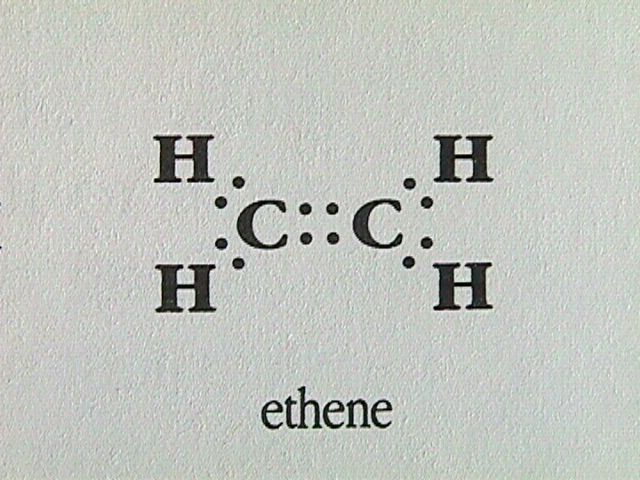
Alkenes contain one or more carbon carbon double bonds.
Alkynes contain one or more carbon carbon triple bonds.

The Shapes of Alkanes:
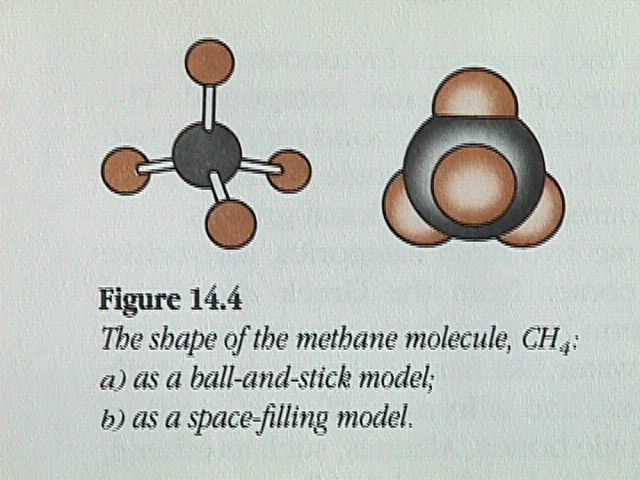
A molecule in which the central atom is surrounded by four electron pairs adopts a tetrahedral arrangement. The tetrahedral shape, with the bond angles of 109.5 degrees, is common among organic compounds.
The simplest alkane molecule is methane.
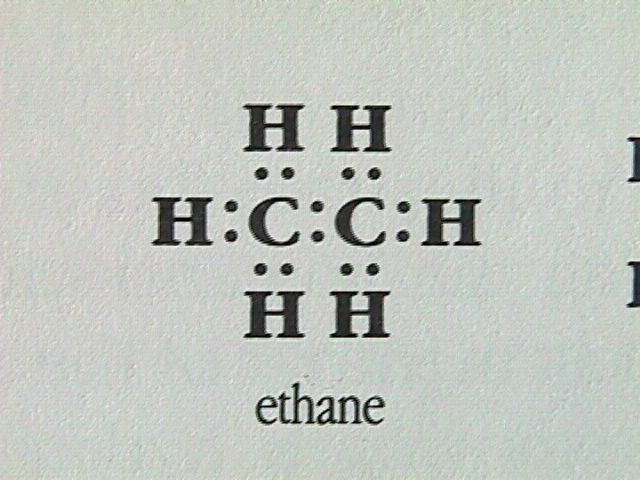
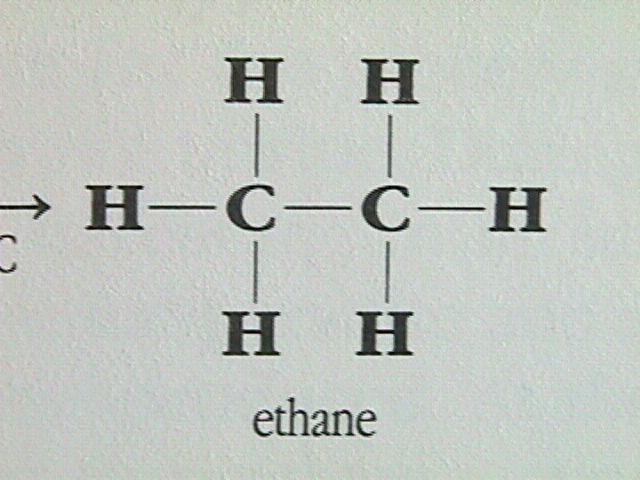
The next simplest member of the alkanes is ethane.
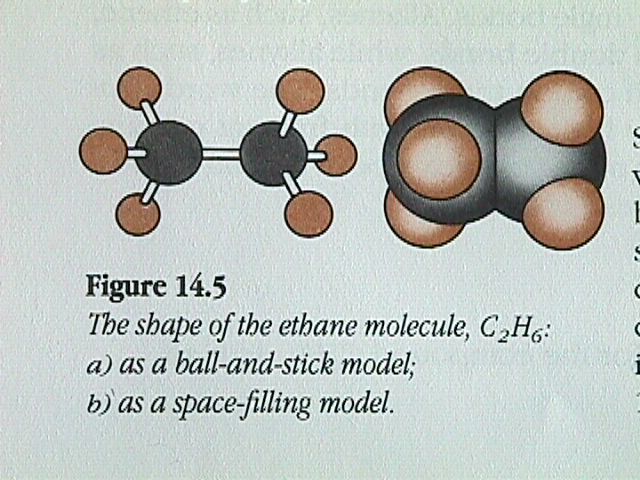
Ethane can be formed by first removing a hydrogen atom from two methane molecules, then combining the two methyl fragments, CH , to restore the octet around each carbon atom. The arrangement of the four electron pairs around each carbon in the ethane molecule will still be tetrahedral.

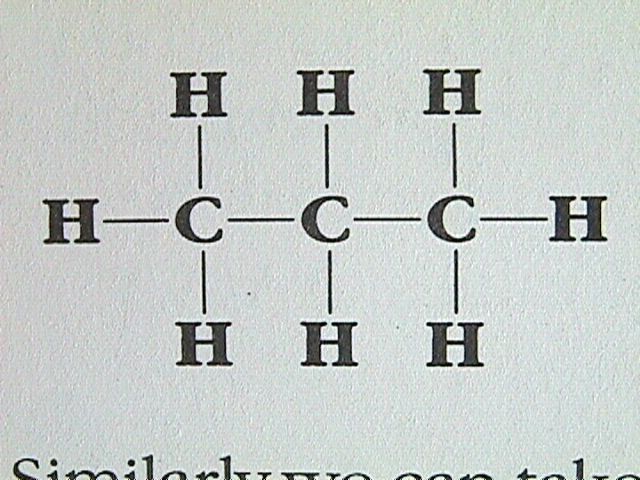
Similarly, we could remove a hydrogen atom from ethane and replace it with another CH fragment to form propane, C H . This procedure can be repeated again and again to produce C H , C H , C H , etc. Because the angle between one carbon bonded to two others is always the tetrahedral angle of 109.5 degrees, a chain of carbon atoms will not form a straight line, but will be arranged in a zigzag manner.
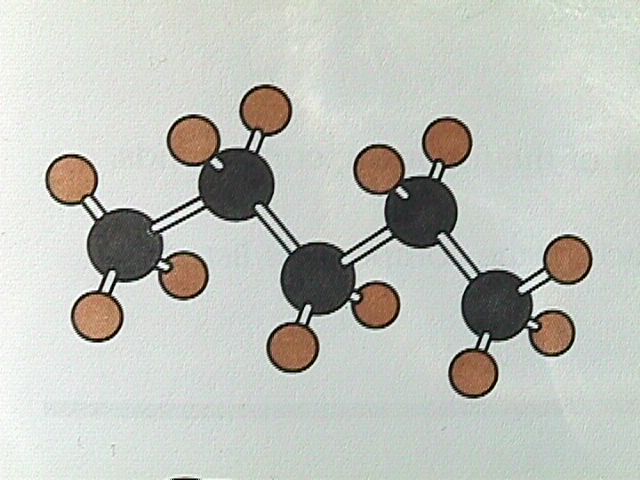
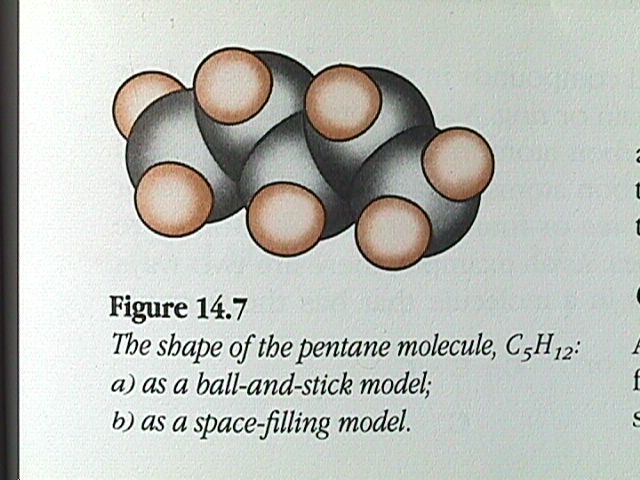
The ball and stick model is projected onto a 2D surface. The 2D representation makes the H C H angles appear to be 90 degree, although the bond angles have the tetrahedral values of 109.5 degrees.
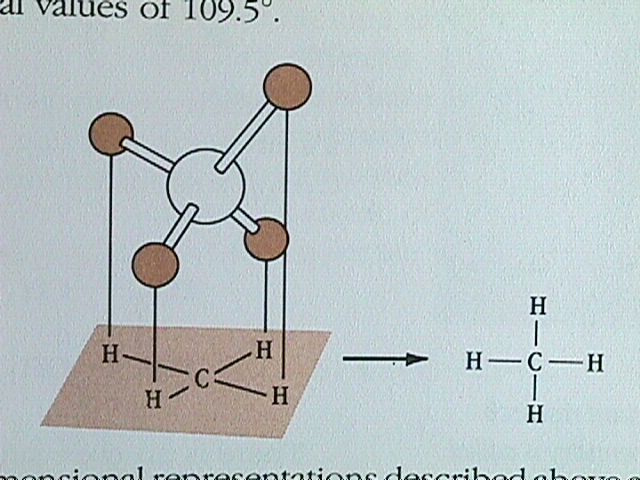
The 2D representation is called a structural formula.
An even more compact form is called a condensed formula. The hydrogen atoms are written beside the carbon atom to which they are attached. When dealing with condensed formulas, it is important to remember that it is the carbon atoms that are bonded together even though this is not apparent from the formulas. The condensed formulas of pentane is
CH-CH-CH-CH-CH
As a further simplification, the lines representing bonds may be omitted and similar units summed together
CH (CH ) CH
Cyclic Hydrocarbons:
A large number of organic compounds contain carbon atoms joined to form rings. When hydrogen and carbon are the only elements involved, such ring compounds are called cyclic hydrocarbons. An example can be constructed from CH CH CH by removing a hydrogen atom from each end. The end carbon atoms can then be joined to form a three membered ring:
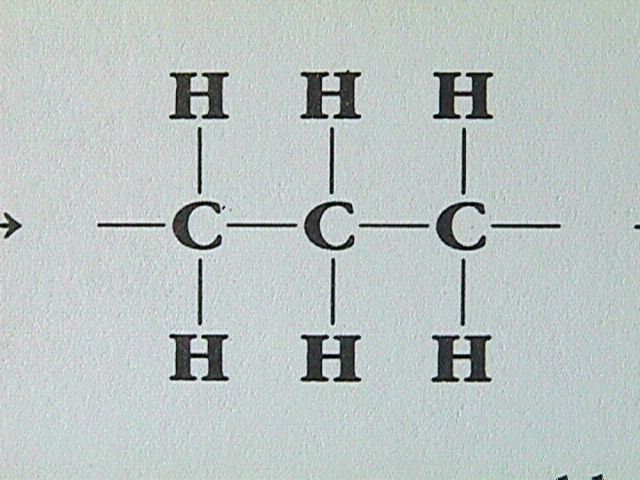
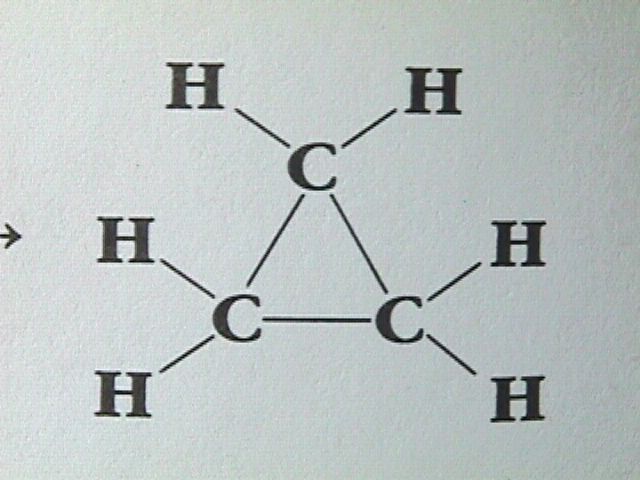
Similarly we can take C H , remove two end hydrogen atoms and create a four member ring. Likewise, a five membered ring can be created from C H , a six membered ring from C H , and so on.
A simpler way of depicting ring structures is by removing the CH2 symbols but retaining the lines of the carbon carbon bonds. The corners mark the location of a CH unit.
Structural Isomers:
Alkanes in which one or more carbon atoms are bonded to three or four other carbon atoms are known as branched chain alkanes.
Compounds that have the same molecular formula, but different structures, are called structural isomers. Such compounds always have different physical properties and often have very different chemical properties.
Condensed formulas for C5H follow:
CH3-CH2-CH2-CH2-CH3
CH CH CH CH
CH C CH
A complex set of mathematical expressions is used to calculate how many isomers are possible for a particular number of carbon atoms. The number of possible isomers increases very rapidly as the number of carbon atoms increases. Isomerism also occurs in cyclic systems.
The Naming of Alkanes:
A systematic method of naming organic compounds has been established by the International Union of Pure and Applied Chemistry (IUPAC). The IUPAC system is based on a series of rules that enables us to deduce the structure of a compound from its name. Conversely, we can write an IUPAC or systematic name for every organic compound. The name indicates both the number and arrangement of the carbon atoms, and the type(s) of functional group(s) in the compound.
Straight Chain Alkanes:
In systematic naming, we rely on the use of prefixes to indicate the number of carbon atoms in a structure.
1=meth
2=eth
3=prop
4=but
5=pent
6=hex
7=hept
8=oct
9=non
10=dec
How to name a straight chain alkane:
CH CH CH CH CH CH CH
1. Since have carbon carbon bonds which are single the suffix ane is used.
2. Count the number of carbon atoms in the chain and choose the appropriate prefix.
There are 6 carbons, so the prefix is hex.
Therefore the compound's name is hexane.
The Six Simplest Straight Chain Alkanes:
methane = CH
ethane = CH CH
propane = CH CH CH
butane = CH (CH ) CH
pentane = CH (CH ) CH
hexane = CH (CH )4CH
Branched Chain Alkanes:
How to name a branched chain alkane:
CH CH CH CH CH CH CH
1. Since have carbon carbon bonds which are single the suffix ane is used.
2. Identify the longest continuous chain of carbon atoms in the molecule. We name this chain according to the method for a straight chain alkane, and refer to this as the base name.
The longest chain has 7 carbons, so the base name is heptane.
3. Then name any side chains or substituents. The names of the substituents are written in front of the base name. A system of numbers is used to identify where the substituents are located along the main chain. The prefixes for the side chains are the same as those of the alkane from which they are derived. As it is a substituent, the ending ane is replaced by yl.
Number the carbon atoms along the main chain, beginning at the end which will result in the lowest possible numbers for the substituents.
Alkyl groups are located at carbon 2 and 4.
4. Name each substituent, and indicate its location on the main chain.
Methyl at carbon 2, ethyl at carbon 4.
5. List the substituents in alphabetical order. Separate the numbers and substituents by hyphens.
Therefore the compound's name is 4 ethyl 2 methylheptane.
If the same substituent occurs more than once in a compound, the quantity of identical groups is indicated by the prefixes
two = di
three = tri
four = tetra
five = penta
six = hexa
seven = hepta
A numerical location must still be given for each substituent, and the numbers are separated by commas.
CH C CH CH CH CH CH
Three methyl groups, two at carbon 2, one at carbon 5.
Therefore the compound's name is 2,2,5 trimethylheptane.
Cycloalkanes:
We identify the presence of a ring by the prefix cyclo.
1. Identify the ring of carbon atoms and assign the base name.
Since the ring has 5 carbons, the base name is cyclopentane.
2. Number the ring beginning with the location which will result in the lowest possible numbers for the substituents.
Alkyl groups at carbon 1 and carbon 3.
. Name each substituent and indicate its position by the number of the carbon atoms on the ring to which it is attached.
Methyl at carbon 1, methyl at carbon 3.
4. Separate the numbers from each other by commas and the numbers from the substituents by hyphens.
Therefore the compound's name is 1,3 dimethylcyclopentane.
The Properties of Alkanes
Because carbon and hydrogen have very similar electronegativities, alkane molecules are essentially nonpolar. The only attraction between neighbouring molecules is by dispersion forces. For straight chain alkanes, the dispersion forces increase with increasing number of carbon atoms in the molecule. The straight chain alkane molecules are basically cylindrical, but the length of the cylinder increases as the number of carbon atoms increases.
A series of compounds that differ in structure by the addition of the same structural unit is called a homologous series.
Boiling Point:
The stronger the intermolecular force, the higher the boiling point, since more energy is need to separate the molecules into the gaseous state. There is a steady increase in boiling point with the length of the carbon chain. The increase in boiling point can be related to the increase in the number of electrons caused by addition of CH units to the chain.
Branched chain alkanes have lower boiling points than straight chain molecules with the same number of carbon atoms. The more compact shape leads to weaker dispersion forces and therefore a lower boiling point.
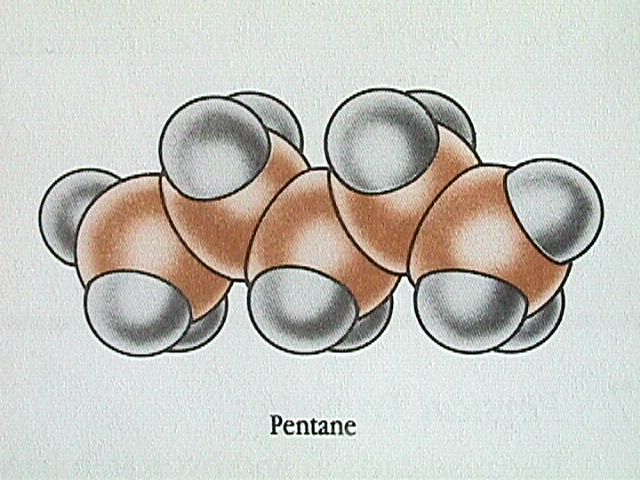
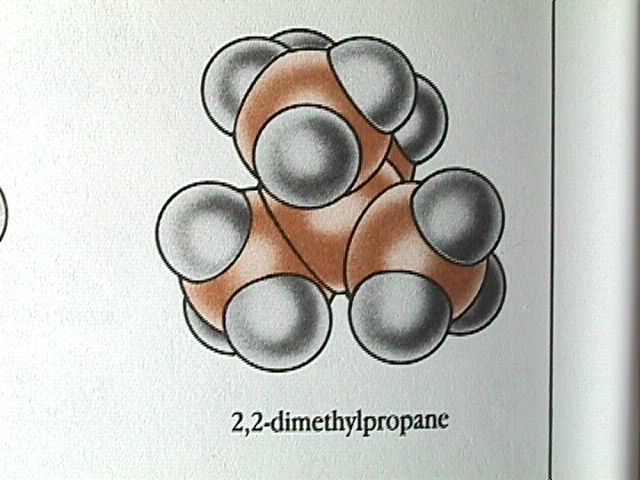
Density:
Density is also related to intermolecular forces. The stronger the intermolecular forces, the more closely neighbouring molecules are attracted to one another, and the higher the density. Since alkanes have weak dispersion forces they have low densities and therefore are less dense than water. As the number of carbon atoms in the alkane chain increases, the density slowly increases. The nonpolar nature of alkanes also accounts for their immiscibilty with water and other solvents.
Chemical Properties:
Alkanes have low chemical reactivity. There is a high activation energy acting as a barrier to their reaction. For nonpolar molecules, ion formation is difficult or impossible, and a defferent reaction mechanism has to operate. The mechanism involves breaking the strong covalent bonds, and thus requires a high activation energy.
Reaction with Oxygen:
Because of their high activation energy, alkanes do not burn sponaneously upon contact with oxygen. A spark or other high energy source is required to initiate the reaction. Provided there is enough oxygen, the products are carbon dioxide and water.
CH4 + O ® CO + H O
Reaction with Chlorine:
The reaction of an alkane with chlorine, as in the reaction with oxygen, involves a high activation energy. The reaction proceeds stepwise, with one hydrogen after another being replaced by chlorine. A reaction in which one atom is replaced by another is called a substitution reaction.
CH4 + Cl ® CH Cl + HCl
CH Cl + Cl ® CH Cl + HCl
CH Cl + Cl ® CH Cl + HCl
CHCl + Cl ® CH Cl4 + HCl
Cracking Reactions
When alkanes are heated in the absence of air, some of the carbon carbon bonds will break. This process, known as cracking, is accelerated by the use of aluminum oxide as a catalyst. Any carbon carbon bond along the chain can break, leading to a large variety of products of shorter chain length.
CH CH CH CH ® CH CH + CH CH
The Preparation of Alkanes
The alkanes of lower molar mass, from methane through pentane, are obtained by separating the components of crude oil. Methane can be prepared by strongly heating a mixture of calcium acetate and calcium hydroxide.
The other alkanes can be prepared by reaction with hydrogen gas in the presence of a platinum or palladium catalyst.
Alkenes:
Hydrocarbons containing one or more carbon carbon double bonds are called alkenes. These compounds and compounds containing carbon carbon tripple bonds are classified as unsaturated hydrocarbons. Alkanes, in which all the bonds are single bonds, are referred to as being saturated hydrocarbons.
Alkenes are nonpolar. The boiling points of alkenes increase with chain length. The boiling point of a particular alkene is slightly less than that of the alkane having the same chain length. Thus the lower members of the alkene series are gases at room temperature.
The presence of a double bon provides a centre for reaction. Thus alkenes are chemically more reactive than alkanes and they undergo different types of reactions.
For alkanes, all the bond angles have the tetrahedral value of 109.5 degrees. In alkenes, the double bond results in only three bonding directions around each carbon atom, and therefore the bond angle is 120 degrees.
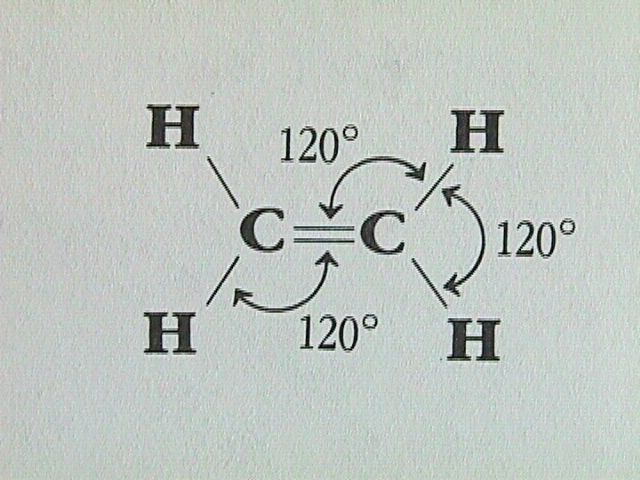
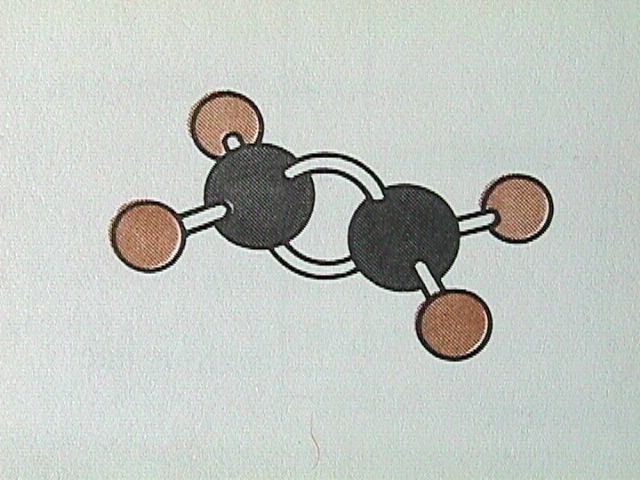
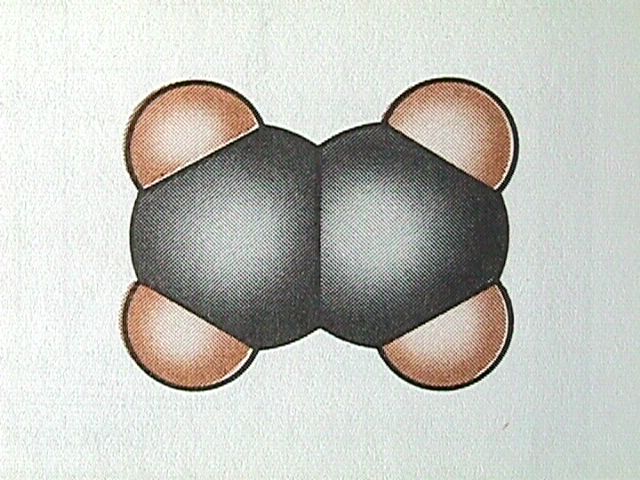
The Naming of Alkenes
CH=C-CH-CH-CH-CH
1. Since have carbon carbon bonds which are double the suffix ene is used. If structural isomers are possible, a number is used to indicate at which carbon atom the double bond starts. This number should be as low as possible. If two double bonds are present, the ending diene is used and two numbers are required to specify the positions of the double bonds. Three double bonds in a molecule are indicated by the ending triene. In this case three numbers are required to specify the location of the double bonds.
2. Identify the longest continuous chain of carbon atoms containing the double bond and assign the base name. (The carbon chain chosen as the main chain must contain the double bond.)
The longest chain containing C=C is six carbons, so the base name is hexene.
3. Number the main chain beginning at the end which will result in the lowest possible number for the double bond.
The double bond starts at carbon 2, hence 2 hexene.
4. Identify each substituent and indicate its position by the number of the carbon atom on the main chain to which it is attached.
A methyl group is at carbon 2 and at carbon 5.
Therefore the compound's name is 2,5 dimethyl 2 hexene.
The Simplest Alkenes:
ethene = CH= CH
propene = CH= CH-CH
1 butene = CH= CH-CH-CH
2 butene = CH-CH=CH-CH
1,3 butadiene = CH=CH-CH=CH
Geometric Isomerism
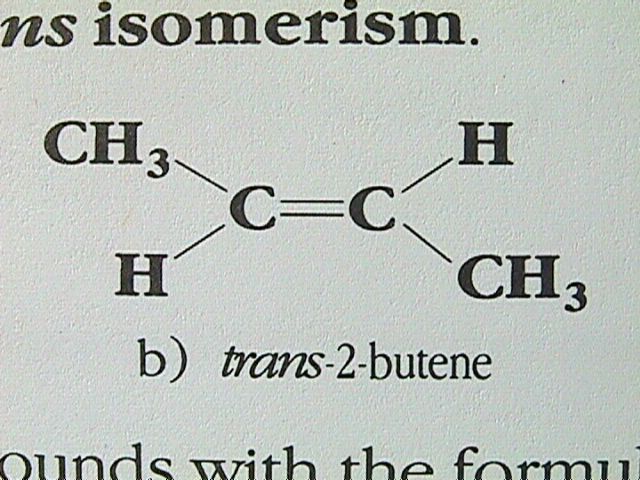
In alkanes, there is free rotation of the molecule about the single bonds. In alkenes, the carbon carbon double bond is quite rigid, preventing any rotation. If each carbon in the double bond has two different groups attached to it, it is possible to have geometric isomers. The simplest alkene in which this type of isomerism can occur is 2 butene. The molecule with both methyl groups on the same side of the double bond is named cis 2 butene, while the isomer with the methyl groups diagonally across the double bond is called trans 2 butene. This type of isomerism is given the name cis trans isomerism.
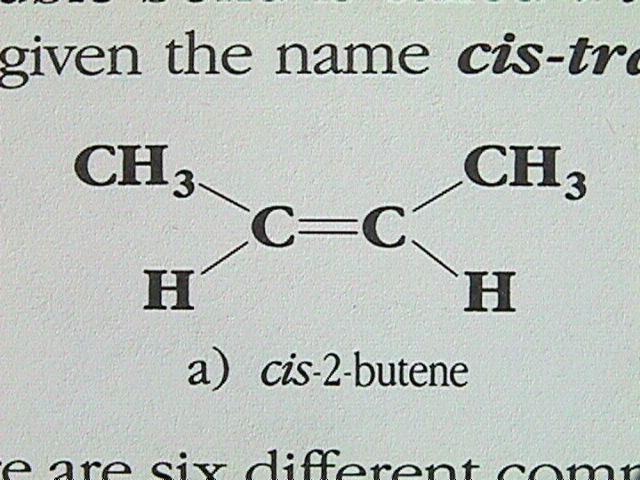
Reactions of Alkenes
The most important reactions for alkanes are substitution reactions, where one atom is replaced by another. Alkenes generally undergo reactions in which atoms or groups of atoms add across the double bond called addition reactions. In the double bond, the two bonds are not equivalent. One is a sigma bond, same as the carbon carbon bond in an alkane. The second bond is a pi bond. It is the pi bond that is broken in addition reactions. Its weakness, in comparison to the sigma bond, accounts for the greater reactivity of alkenes.
Common Addition Reactions
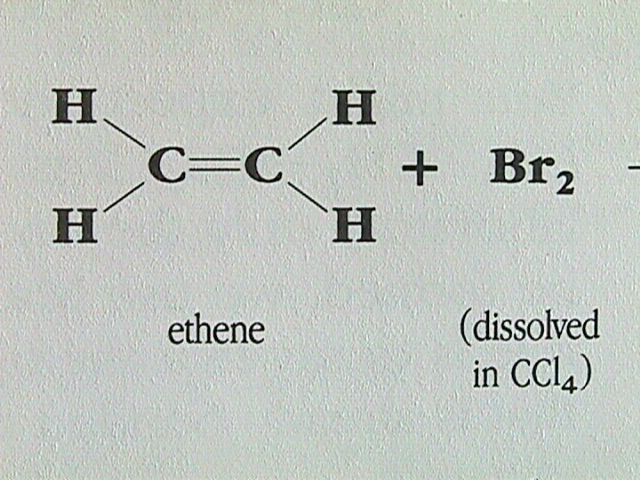
Reaction with Halogens: Alkenes react rapidly with halogens such as chlorine and bromine.
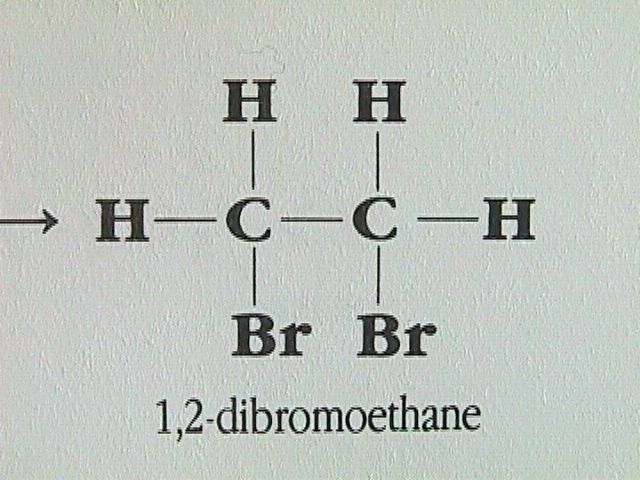
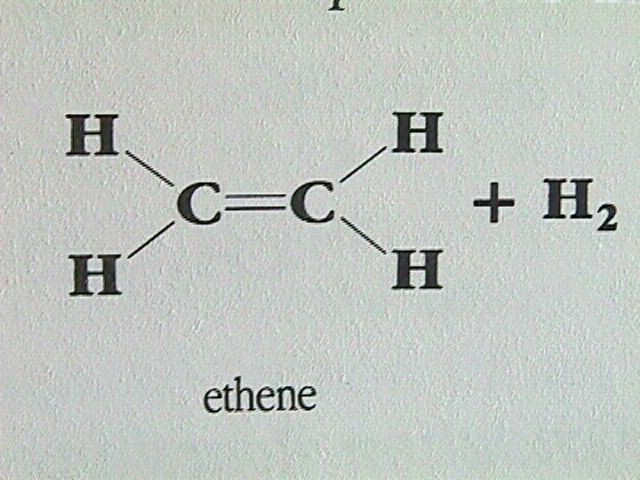
Reaction with Hydrogen:A similar reaction occurs between an alkene and hydrogen gas. This process is called hydrogenation. It is used in the preparation of margarine. The hydrogenation of some of the double bonds produces a less unsaturated compound with a slightly higher melting point.
Reaction with Hydrogen Halides: Alkenes also react with hydrogen halides, eg. hydrogen chloride reacts with ethene to produce chloroethane, C H5Cl.
To predict which isomer will be formed in an addition reaction between an alkene and hydrogen halides we use Markovnikov's Rule. The hydrogen of a hydrogen halide adds to the carbon that is already bonded to the most hydrogen atoms.
Reaction with Water: In acidic solution, alkenes undergo an addition reaction with water to produce an alcohol.
Oxidation Reactions: Alkenes, like alkanes, can be oxidized by burning them in air.
Polymerization Reactions: From the perspective of the chemical industry, the single most important reaction of alkenes is polymerization. A polymer is a large organic molecule that consists of many small, identical units. An example of a polymerization reaction is the formation of polyethene from ethene. IN this reaction the carbon carbon double bond is broken and new single bonds are formed between neighbouring ethene molecules. In a typical polymerization reaction, between 2000 and 50 000 alkene units may become bonded together. To represent this, we place the symbol n outside the parenthesis that contains the repeating unit.
The Biochemistry of Ethene:
Ethene gas plays an important role in the ripening of fruit. At the onset of the ripening phase, enzymes in the fruit start producing ehtene.
Preparation of Alkenes:
Alkenes can be prepared in the lab from the dehydration of an alcohol. A dehydration reaction is one in which the elements of water (hyrdrogen and oxygen) are removed from a compound in a 2:1 mole ratio. In the lab preparation of ethene, we can alternatively pass ethanol vapour over heated alulminum oxide.
Alkynes:
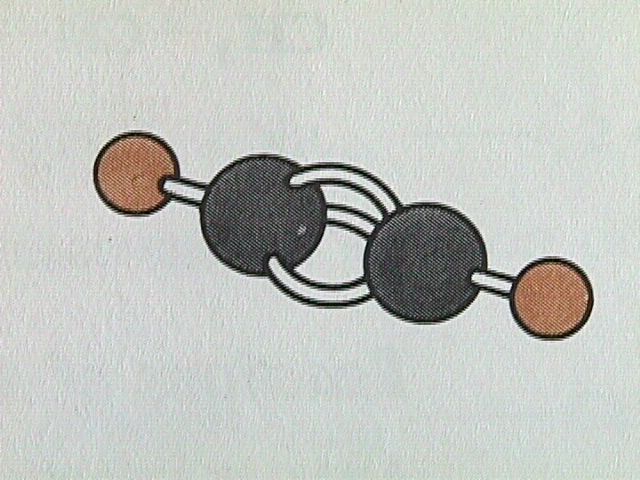
Hydrocarbons containing one or more triple bonds are called alkynes. Being unsaturated compounds, alkynes undergo basically the same types of reactions as alkenes. SInce each carbon of the triple carbon carbon bond can only bond to one other atom, alkynes do not exhibit geometrical isomerism, only structural isomerism. The linear arrangement of atoms about the carbon carbon triple bond can be seen in the simplest alkyne, ethyne.
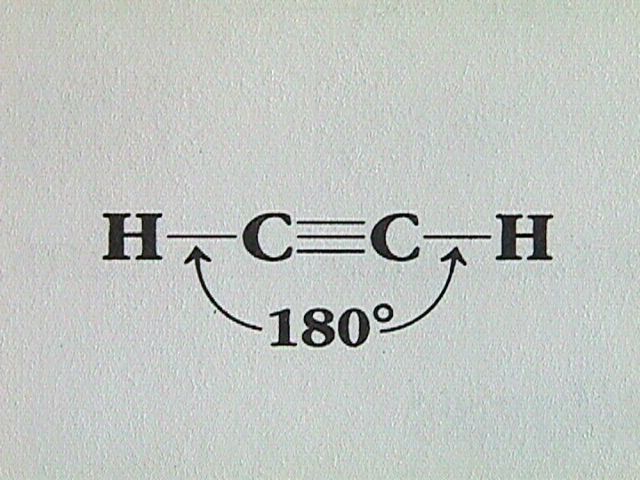
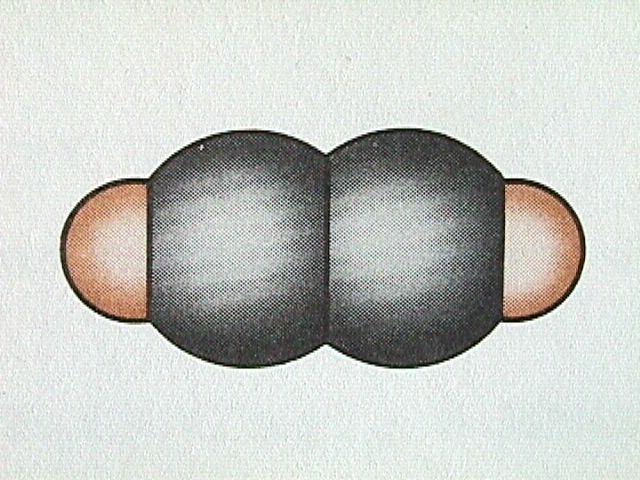
The Naming of Alkynes:
CH-CH-CH-C-C-CH-CH
1. Since have carbon carbon bonds which are triple the suffix yne is used.
2. Identify the longest continuous chain of carbon atoms containing the triple bond and assign the base name.
The longest chain containing C- C is seven carbons, so the base name is heptyne.
3. Number the main chain beginning at the end which will result in the lowest possible number for the triple bond.
The triple bond starts at carbon 3, hence 3 heptyne.
4. Name each substituent and indicate its position by the number of the carbon atom on the main chain to which it is attached.
A methyl group is at carbon 2 and at carbon 6.
Therefore the compound's name is 2,6 dimethyl 3 heptyne.
The Simplest Alkynes:
ethyne = CH- CH
propyne = CH- CCH
1 butyne = CH- CCH CH
2 butyne = CH- C- C-CH
Reactions of Alkynes:
Alkynes behave like alkenes in that they undergo addition reactions. However, on e major difference is that it is possible for two moles of reagent to add to one mole of an alkyne. Two moles of hydrogen react with one mole of ethyne to produce the corresponsing alkane:
Similarly, in the common test for an unsaturated carbon carbgon bond, two moles of bromine add to one mole of an ethyne.
In another reaction parallel to that of alkenes, a hydrogen halide will add to a triple bond.
The Acidity of Alkynes:
Although the carbon hydrogen bond is normally of very low polarity, we find that the carbon hydrogen bond next to a carbon carbon triple bond is quite polar. As a result, 1 alkynes can behave as very weak acids.
Preparation of Ethyne:
Ethyne produced by converting limestone (calcium carbonate) to calcium oxide, and the coal is converted to coke (impure coal). The two products are then reacted together to produce calcium carbide, CaC . Treatment of calcium carbide with water produces ethyne gas.