HEXAGONS
in a packed World
|
| |
|
GONSHEXAGONS
HEXAGONSHEXA
GONSHEXAGONS
HEXAGONSHEXA
GONSHEXAGONS |
Hexagons are everywhere!. Everywhere
you can find a bunch of homogeneous objects that is. Whether we look into
beehives, man-made devices, living tissues or even atoms we can find surprising
examples of a characteristic hexagonal pattern. We call it hexagonal close
packing and it is in fact most effective to pack the largest number of
objects in a minimum space. Let us see some examples.
|
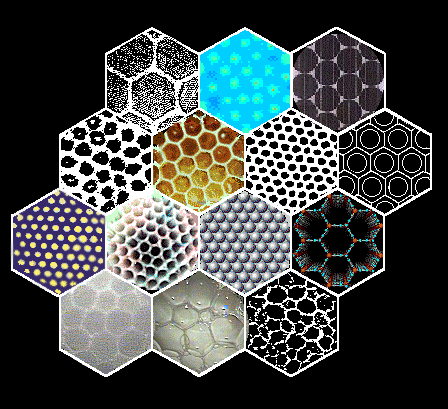
| Each of the hexagonal cells on the right shows an
example of hexagonal pattern.
Some are natural, some man-made. Keep reading and you will run into
many surprises, as well as enlightening similarities and differences.
I would suggest following the numbered sequence, beginning with the
familiar beehive honeybee pattern below(1).
|
 |
1
 ¿ Have you ever wondered why bees make their beehives in their characteristic
honeybee pattern?. As you know, each cell will harbor one larva on its
way to become a bee. As we will see, the typical "honeycomb" hexagonal
arrangement is the most efficient way to pack as many cells as possible
in a limited space.
¿ Have you ever wondered why bees make their beehives in their characteristic
honeybee pattern?. As you know, each cell will harbor one larva on its
way to become a bee. As we will see, the typical "honeycomb" hexagonal
arrangement is the most efficient way to pack as many cells as possible
in a limited space.
By the way, did you know that bees make each cell cylindrical,
like a tube?. The fact that the cells look hexagonal is due to their compression
by the six closest neighbors in the crowded colony!. |
| It is something similar to
what happens to bubbles when they get together. Isolated bubbles are spherical
but look how they look like when they stick together. |
 |
2
 |
We humans have also managed to figure out how
to pack disks, cylinders or spheres efficiently. Those on the left are
single-crystal Silicon wafers (thin disks) arranged in a photoconversion
panel for harvesting solar energy. Again efficient hexagonal packing. |
3
 We
are able to close-pack disks in two dimensions and also spheres in three
dimensions. Piles of fruit are a tasty example. Each layer of oranges in
this pile is close-packed as the silicon disks above. As we move up we
put each new orange sitting on the middle point between three bottom oranges,
and... we end up with hexagonal close packing along the vertical direction
too!. Try it at home. If you don't have oranges cannonballs will also do
: ) Could you guess how many oranges would be directly in contact with
an orange inside this pile? We
are able to close-pack disks in two dimensions and also spheres in three
dimensions. Piles of fruit are a tasty example. Each layer of oranges in
this pile is close-packed as the silicon disks above. As we move up we
put each new orange sitting on the middle point between three bottom oranges,
and... we end up with hexagonal close packing along the vertical direction
too!. Try it at home. If you don't have oranges cannonballs will also do
: ) Could you guess how many oranges would be directly in contact with
an orange inside this pile? |
4
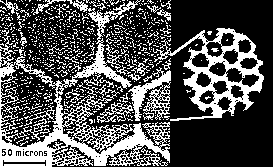 So
far we have seen examples of hexagonal arrangements of macroscopic
objects. But the microscopic world is also full of interesting examples.
The image on the left is a micrograph of a cut of a superconducting cable.
This cable is made of many fibers wich in turn are made of many tinier
filaments (seen as small dots). The scale on the bottom marks the size
of 50 microns (1 micron is 1/1000 millimeters), so that a 0.5 mm thick
cable would span almost a full (14") screen. The larger fibers, with radius
of aproximately 50 microns pack together very much like cells in a beehive.
In this case the fibers were also little cylinders to begin with. But the
process of fabricating the cable, by forcing it to pass through a series
of size-reducing funnels forces the fibers against each other and the familiar
honeycomb pattern results. Now, if we look closer inside each fiber we
can
see even smaller filaments each made of superconducting niobium which have
also SPONTANEOUSLY packed into a close-packed hexagonal array !. So
far we have seen examples of hexagonal arrangements of macroscopic
objects. But the microscopic world is also full of interesting examples.
The image on the left is a micrograph of a cut of a superconducting cable.
This cable is made of many fibers wich in turn are made of many tinier
filaments (seen as small dots). The scale on the bottom marks the size
of 50 microns (1 micron is 1/1000 millimeters), so that a 0.5 mm thick
cable would span almost a full (14") screen. The larger fibers, with radius
of aproximately 50 microns pack together very much like cells in a beehive.
In this case the fibers were also little cylinders to begin with. But the
process of fabricating the cable, by forcing it to pass through a series
of size-reducing funnels forces the fibers against each other and the familiar
honeycomb pattern results. Now, if we look closer inside each fiber we
can
see even smaller filaments each made of superconducting niobium which have
also SPONTANEOUSLY packed into a close-packed hexagonal array !. 
That is the way in which a given number of filaments can get to occupy
the smallest space.
If we take a bunch of straws, as in the picture on the right, or pencils
or toothpicks, and hold them tightly together with a rubber band that is
precisely what we get: hexagonal close packing. |
5
Up to this point we have seen a few examples of human-made
(also bee-made) close-packed structures. It might seem that these arrays
are a signature of intelligence (we could always think that bees are pretty
inteligent too, after all they are even able to orient themselves and comunicate
directions!).
Yet, we will soon see amazing examples of hexagonal packing in unsuspected
places that will rule out this self-indulging hypothesis.
 This
picture represents a section of a sensitive part of the retina called the
fovea ("Yellow Spot"). The fovea is a small (less than 1 square mm)
but very specialized region of the retina. There the sampling of light
is at its finest. It consists of discrete cellular elements: very thin
(3 µm) elongated cone photoreceptors (rod-shaped but seen here as
black spots). The mosaic of foveal cones is highly condensed (200.000 cones/sq.mm
in the adult human ) providing maximum This
picture represents a section of a sensitive part of the retina called the
fovea ("Yellow Spot"). The fovea is a small (less than 1 square mm)
but very specialized region of the retina. There the sampling of light
is at its finest. It consists of discrete cellular elements: very thin
(3 µm) elongated cone photoreceptors (rod-shaped but seen here as
black spots). The mosaic of foveal cones is highly condensed (200.000 cones/sq.mm
in the adult human ) providing maximum
resolution of space, contrast and color. And how are these rod-cells
arranged ?. You got it !.
They cover the space most efficiently adopting a hexagonal (honeycomb)
pattern (take a stack of round toothpicks for example and look at their
tips). In this case we find the pattern at the cellular level. These are
truly self-assembled complex tissues.
To finish this section, here is a cute similarity between
natural and man-made devices: Photosensitive panels used in man-made night
vision devices are also built with close-paked hexagonal arrays |
6
 If you thought self-assembly of cells was amazing wait until you read the
next one.
If you thought self-assembly of cells was amazing wait until you read the
next one.
After all, cells can assemble according to preordained instructions
coded in DNA, right?. Nothing to it.
But what if I tell you that certain chemical
reactions, kept far from their thermodynamic equilibrium, can evolve into
ordered heterogeneous sytems with hexagonal symmetry ?. It would be as
amazing as pouring ink into water and eventually getting a spotty mixture
!. Well, something like that is what happens in the case of the figure
on the left. A reaction in a continuously fed reactor between chlorite
and iodide in the presence of malonic acid (CIMA reaction for short) can
go back and forth. Coupled with molecular diffusion and evolving far from
equilibrium, this reversible reaction leads to iodide-rich (blue)
and iodide-poor (yellow) regions which ORDER SPONTANEOUSLY into our already
familiar hexagonal symmetry.
And these funny chemical reactions are full of other surprises.
The hexagonal pattern is the most stable of the two, but only small variations
in the concentration of reagents in the same CIMA  reaction,
in the same reactor, can lead to the radically different pattern on the
right. reaction,
in the same reactor, can lead to the radically different pattern on the
right.
Aren't these patterns awfully familiar ?. We can certainly found them
in Nature, here and there. I will put some relevant pictures here as samples
but probably you could find some more. (Point to each one to find out their
true nature).
     
Probably more than amazing similarities. Don't you think?
|
7
And now... we will get into the atomic realm.
Yes, there too we will find hexagonal close-packing. As
a matter of fact it is a classical example.
If we consider atoms as single spheres then there
is no surprise that when many of them get together (for example in metals
at room temperature or certain gases at very low temperature) they arrange
as shown in this figure. Not only neutral atoms pack in this way. Ions
(charged atoms) also do, as long as their charge could be effectively neutralized
by other ions of opposite charge sitting in some of the intersticial sites
of the structure (small triangular cavities in the figure).
 And how do we know that they arrange themselves like that?. Atoms are so
small that they cannot be seen with microscopes (although High-Resolution
Electron Microscopes are getting close to it). But scientists devised a
trick to "see" atoms and molecules in crystals by shining X-rays onto them
and measuring the rays reflected by the crystal in each direction of space.
This is X-ray crystallography, amounts to what we could call "X-ray vision"
and lets us know that indeed in many elements, atoms are arranged as shown
here but in the three dimensions of space. That is something like in the
pile of oranges shown above, in section 3.
And how do we know that they arrange themselves like that?. Atoms are so
small that they cannot be seen with microscopes (although High-Resolution
Electron Microscopes are getting close to it). But scientists devised a
trick to "see" atoms and molecules in crystals by shining X-rays onto them
and measuring the rays reflected by the crystal in each direction of space.
This is X-ray crystallography, amounts to what we could call "X-ray vision"
and lets us know that indeed in many elements, atoms are arranged as shown
here but in the three dimensions of space. That is something like in the
pile of oranges shown above, in section 3. |
8
After all these examples it looks as
if close packing could be found anywhere!. Well, almost anywhere. But not
quite; you need crowding of similar homogeneous objects, whether atoms,
cells or oranges. We do not find this packing in stars or galaxies, far
away from each other. But certainly in our packed world it is possible
to find this recurrent pattern in many different places and at many different
scales.
Since ours is a complex world, not always things will
be as straighforward as the examples shown here. Let me show you some of
the exceptions, complications and variability that we can find in Nature.
Sometimes we can find perfectly hexagonal patterns
which are not the result of close packing.
Ice crystals in snow flakes for instance are always
hexagonal and this geometry can be traced back to the arrangement of the
molecules of water frozen into a hexagonal structure (See the related story
Snow
flakes). But this symmetry is the result of the geometry imposed by
the chemical bonds in the lattice of ice. It is the atoms making the crystal
and how they "like" to bond to each other that determine the hexagonal
arrangement in this case.
Something similar happens in the case of graphite, a black
form of carbon used as a powder in the core of pencils.  Graphite
has hexagonal symmetry that derives from the way each Carbon atom shares
electrons through chemical bonds with three nearest neighbors (See
the story Is this a molecule?). Graphite
has hexagonal symmetry that derives from the way each Carbon atom shares
electrons through chemical bonds with three nearest neighbors (See
the story Is this a molecule?).
Sometimes we can find close packing not leading to perfectly hexagonal
arrays.
And this is so because real systems are not always as
ideal as balls in a pool table. In order to have hexagonal close packing
we need isotropic objects all of the same size. If the sizes are different
funny things happen. As in the buble bath below. There we can find medium-size
bubbles surrounded by six other bubbles. But there is also a smaller sized
bubble surrounded by five neighbors. A larger than average bubble would
be obviously surrounded by more smaller friends.
   
The other two pictures next to the bubble bath represent examples of
natural and man-made systems where we can find patterns with complexities
similar to that of foam. The middle pictures are different views of a plastic
very familiar to all of us in the form of expanded polystyrene foam (styrofoam,
the white light stuff used in packaging). This material is made by soaking
polystyrene beads with a solvent of low boiling point (pentane) then heating
quickly so that the solvent is boiled out, the plasric beads expand, growing
into each other and becoming at the same time less dense (more volume for
the same weight). If all the expanded beads were the same size then perfect
packing could be expected. Since there is enough dispersion of sizes we
get a situation similar to the bubble bath. After all it is a foam.
The final image is a micrograph of cork. Also a light-weight material,
but in this case one found in nature. Cork is so light because it is full
of air. It is formed by long empty channels that run perpendicular to the
picture. Again, these channels are densely packed but their different sizes
and irregularities make the pattern less obvious. |
Sooo... we run out of examples of closed-packed hexagonal systems. Or
didn't we?.
Not quite actually.
I will show you now a few more examples and I invite you to let me
know of any other you can think of. I'll include it in the list!
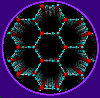
 This
leopard-like pattern shows the distribution of lines of a magnetic field
when they begin to penetrate a superconducting sample. The position of
the lines can be calculated according to models and also detected experimentally
by "decoration" with magnetic particles. In both cases it is shown that
the lines (in the picture cut as brown dots) pack with approximate hexagonal
symmetry
This
leopard-like pattern shows the distribution of lines of a magnetic field
when they begin to penetrate a superconducting sample. The position of
the lines can be calculated according to models and also detected experimentally
by "decoration" with magnetic particles. In both cases it is shown that
the lines (in the picture cut as brown dots) pack with approximate hexagonal
symmetry
 This is a
close-up fotograph of the speakers in my computer. By paking holes as densely
as possible in these grids, dessigners kill two birds with a stone: as
much open space to let sound go through and the smallest amount of material
(in this case plastic) to make the grid. Can you think of any other "net"
with these characteristics. Used in farms maybe?
This is a
close-up fotograph of the speakers in my computer. By paking holes as densely
as possible in these grids, dessigners kill two birds with a stone: as
much open space to let sound go through and the smallest amount of material
(in this case plastic) to make the grid. Can you think of any other "net"
with these characteristics. Used in farms maybe?
 Chicken
wire, yes.
Chicken
wire, yes.
 The
End
The
End
Send your comments or examples of other hexagonal packings to science4all@iname.com
Last modified: 25 March,1999
©Pedro Gómez-Romero, 1999
 Back to main page on Science Technology and Society
Back to main page on Science Technology and Society

 ¿ Have you ever wondered why bees make their beehives in their characteristic
honeybee pattern?. As you know, each cell will harbor one larva on its
way to become a bee. As we will see, the typical "honeycomb" hexagonal
arrangement is the most efficient way to pack as many cells as possible
in a limited space.
¿ Have you ever wondered why bees make their beehives in their characteristic
honeybee pattern?. As you know, each cell will harbor one larva on its
way to become a bee. As we will see, the typical "honeycomb" hexagonal
arrangement is the most efficient way to pack as many cells as possible
in a limited space.


 We
are able to close-pack disks in two dimensions and also spheres in three
dimensions. Piles of fruit are a tasty example. Each layer of oranges in
this pile is close-packed as the silicon disks above. As we move up we
put each new orange sitting on the middle point between three bottom oranges,
and... we end up with hexagonal close packing along the vertical direction
too!. Try it at home. If you don't have oranges cannonballs will also do
: ) Could you guess how many oranges would be directly in contact with
an orange inside this pile?
We
are able to close-pack disks in two dimensions and also spheres in three
dimensions. Piles of fruit are a tasty example. Each layer of oranges in
this pile is close-packed as the silicon disks above. As we move up we
put each new orange sitting on the middle point between three bottom oranges,
and... we end up with hexagonal close packing along the vertical direction
too!. Try it at home. If you don't have oranges cannonballs will also do
: ) Could you guess how many oranges would be directly in contact with
an orange inside this pile? So
far we have seen examples of hexagonal arrangements of macroscopic
objects. But the microscopic world is also full of interesting examples.
The image on the left is a micrograph of a cut of a superconducting cable.
This cable is made of many fibers wich in turn are made of many tinier
filaments (seen as small dots). The scale on the bottom marks the size
of 50 microns (1 micron is 1/1000 millimeters), so that a 0.5 mm thick
cable would span almost a full (14") screen. The larger fibers, with radius
of aproximately 50 microns pack together very much like cells in a beehive.
In this case the fibers were also little cylinders to begin with. But the
process of fabricating the cable, by forcing it to pass through a series
of size-reducing funnels forces the fibers against each other and the familiar
honeycomb pattern results. Now, if we look closer inside each fiber we
can
see even smaller filaments each made of superconducting niobium which have
also SPONTANEOUSLY packed into a close-packed hexagonal array !.
So
far we have seen examples of hexagonal arrangements of macroscopic
objects. But the microscopic world is also full of interesting examples.
The image on the left is a micrograph of a cut of a superconducting cable.
This cable is made of many fibers wich in turn are made of many tinier
filaments (seen as small dots). The scale on the bottom marks the size
of 50 microns (1 micron is 1/1000 millimeters), so that a 0.5 mm thick
cable would span almost a full (14") screen. The larger fibers, with radius
of aproximately 50 microns pack together very much like cells in a beehive.
In this case the fibers were also little cylinders to begin with. But the
process of fabricating the cable, by forcing it to pass through a series
of size-reducing funnels forces the fibers against each other and the familiar
honeycomb pattern results. Now, if we look closer inside each fiber we
can
see even smaller filaments each made of superconducting niobium which have
also SPONTANEOUSLY packed into a close-packed hexagonal array !. 
 This
picture represents a section of a sensitive part of the retina called the
fovea ("Yellow Spot"). The fovea is a small (less than 1 square mm)
but very specialized region of the retina. There the sampling of light
is at its finest. It consists of discrete cellular elements: very thin
(3 µm) elongated cone photoreceptors (rod-shaped but seen here as
black spots). The mosaic of foveal cones is highly condensed (200.000 cones/sq.mm
in the adult human ) providing maximum
This
picture represents a section of a sensitive part of the retina called the
fovea ("Yellow Spot"). The fovea is a small (less than 1 square mm)
but very specialized region of the retina. There the sampling of light
is at its finest. It consists of discrete cellular elements: very thin
(3 µm) elongated cone photoreceptors (rod-shaped but seen here as
black spots). The mosaic of foveal cones is highly condensed (200.000 cones/sq.mm
in the adult human ) providing maximum
 If you thought self-assembly of cells was amazing wait until you read the
next one.
If you thought self-assembly of cells was amazing wait until you read the
next one.
 reaction,
in the same reactor, can lead to the radically different pattern on the
right.
reaction,
in the same reactor, can lead to the radically different pattern on the
right.






 And how do we know that they arrange themselves like that?. Atoms are so
small that they cannot be seen with microscopes (although High-Resolution
Electron Microscopes are getting close to it). But scientists devised a
trick to "see" atoms and molecules in crystals by shining X-rays onto them
and measuring the rays reflected by the crystal in each direction of space.
This is X-ray crystallography, amounts to what we could call "X-ray vision"
and lets us know that indeed in many elements, atoms are arranged as shown
here but in the three dimensions of space. That is something like in the
pile of oranges shown above, in section 3.
And how do we know that they arrange themselves like that?. Atoms are so
small that they cannot be seen with microscopes (although High-Resolution
Electron Microscopes are getting close to it). But scientists devised a
trick to "see" atoms and molecules in crystals by shining X-rays onto them
and measuring the rays reflected by the crystal in each direction of space.
This is X-ray crystallography, amounts to what we could call "X-ray vision"
and lets us know that indeed in many elements, atoms are arranged as shown
here but in the three dimensions of space. That is something like in the
pile of oranges shown above, in section 3. 




 This
leopard-like pattern shows the distribution of lines of a magnetic field
when they begin to penetrate a superconducting sample. The position of
the lines can be calculated according to models and also detected experimentally
by "decoration" with magnetic particles. In both cases it is shown that
the lines (in the picture cut as brown dots) pack with approximate hexagonal
symmetry
This
leopard-like pattern shows the distribution of lines of a magnetic field
when they begin to penetrate a superconducting sample. The position of
the lines can be calculated according to models and also detected experimentally
by "decoration" with magnetic particles. In both cases it is shown that
the lines (in the picture cut as brown dots) pack with approximate hexagonal
symmetry
 This is a
close-up fotograph of the speakers in my computer. By paking holes as densely
as possible in these grids, dessigners kill two birds with a stone: as
much open space to let sound go through and the smallest amount of material
(in this case plastic) to make the grid. Can you think of any other "net"
with these characteristics. Used in farms maybe?
This is a
close-up fotograph of the speakers in my computer. By paking holes as densely
as possible in these grids, dessigners kill two birds with a stone: as
much open space to let sound go through and the smallest amount of material
(in this case plastic) to make the grid. Can you think of any other "net"
with these characteristics. Used in farms maybe?
 Chicken
wire, yes.
Chicken
wire, yes.
 The
End
The
End