


[Advertised Claims] [Description] [ Commercial Availability and General Use] [Ergogenic Effects] [Risks and Disadvantages] [Recommendations]
| Advertised
Claims |
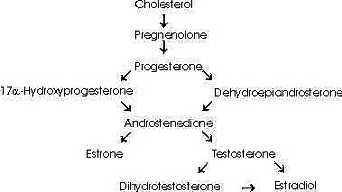
(
See Androstenedione Conversion Table)However, androstenedione may also be converted to the estrogen, estrone, by aromatase (estrogen synthase). Furthermore, testosterone itself may be converted to the estrogen, estradiol, by aromatase or dihydrotestosterone by 5alpha -reductase (6,7).
| Commercial
Availability and General Use Androstenedione supplements are sold in health food stores. However, the use of androstenedione supplements is banned by the International Olympic Committee, the National Football League, and the National Collegiate Athletic Association. It is classified as a dietary supplement. Androstenedione is available in various forms including 4-androstenedione and 5-androstenedione. Analogs of androstenedione also available include 4- or 5-androstenediol and 4- or 5-norandrostene- dione or diol. Androstenedione is typically sold in the form of tablets and capsules but is also available in other forms including percutaneous gels, transdermal patches, and chewing gums. The typical, suggested method of intake is to consume 50-200 mg of androstenedione per day. Many manufacturers of androstenedione suggest that the supplement be consumed about an hour prior to exercise. Also, many manufacturers suggest cycling the use of the supplement (e.g. "four weeks on/one week off). Cost of the supplement is approximately $15-$40 for a 30-day supply. |
Research Trials
Mahesh and Greenblatt
A study conducted in 1962 by Mahesh and Greenblatt (8) reported that two female subjects who ingested 100 mg of androstenedione had dramatically elevated blood testosterone levels within 90 minutes of ingestion. At 60 minutes, serum testosterone concentration increased by approximately 400% in one of the subjects and nearly 700% in the other subject. Serum testosterone concentration remained elevated in both subjects for about 90 minutes before returning to the normal level (8).
However, this study did not examine whether the acute increase in testosterone had any implication regarding muscle strength or size. It should be noted that the elevated levels of testosterone within the subjects were sharply reduced within 1 hour to 90 minutes after ingestion of androstenedione and returned to normal levels after 90 minutes. Furthermore, it should also be noted that the study only involved four female subjects. (Two subjects were treated with DHEA and the other two subjects were treated with androstenedione) (8). Thus, this particular study does not provide sufficient data to suggest that androstenedione supplementation can raise blood testosterone levels in normotestosterogenic men.
German patent
The German patent for androstenedione claims that androstenedione increased testosterone concentration by over 200% in humans and maintained elevated testosterone levels for about a week (11). However, this patent did not provide much of the data necessary to support the validity of its claims.
King et al.
A recent study conducted by Douglas King et al. (5) reported no increased levels of testosterone in normotestosterogenic, male subjects who were supplemented with androstenedione. Ten male subjects aged 19-29 years old orally consumed 300 mg of androstenedione while another 10 subjects consumed a placebo. The subjects underwent a resistance-training program during the study. The study was conducted for 8 weeks and was a double blind, randomized trial. No change in serum free or total testosterone levels were observed in any of the subjects compared to baseline during the 8-week period (5).
Ten additional male subjects participated in an acute trial (testing for an acute hormone response). This trial was a randomized, double-blinded, crossover study with a one-week washout period. The subjects either consumed 100 mg of androstenedione or a placebo after an overnight fast. Blood samples were drawn every 30 minutes for 6 hours. No change in serum free or total testosterone levels were observed during the 6-hour period (5).
However, androstenedione concentration did increase by as much as 100% during the 8-week study in the subjects treated with androstenedione. During the acute trial, serum androstenedione was elevated by about 175% at 60 minutes and 325% to 350% at 90-270 minutes. After 270 minutes, the androstenedione concentration decreased but remained elevated above baseline (5).
This increase in androstenedione occurred concomitantly with a significant increase in estrone and estradiol, indicating that increased concentration of androstenedione and a transient increase in testosterone led to increased aromatization of both androgens into the undesirable estrogens. (More on estrogen and aromatase activity below.) King's study observed no significant differences in strength, lean body mass, or muscle size between the placebo group and the treatment group during the 8-week period.
It should be noted, however, that the subjects were not highly trained (in resistance-training) before the study. Therefore, it is possible that gains attributable to androstenedione supplementation were undetectable in light of the gains in muscle size and strength that tend to occur relatively quickly during the initial phases of resistance-training. Furthermore, the dosage of androstenedione used in this study (300 mg/d) were less than the amount suggested by some of the commercial manufacturers of the supplement (as much as 1200 mg/d) (26).
Furthermore, King's study reported significantly lowered serum concentrations of high-density lipoprotein cholesterol (HDL-C) in the subjects who consumed androstenedione during the 8 week study (5). This finding is consistent with previous studies that reported similar results in subjects treated with other androgenic-anabolic steroids (though the changes in blood lipid levels were much greater in the subjects taking the illegal, androgenic-anabolic steroids) (5,22). Reduced HDL-C is associated with an increased risk of cardiovascular disease (23).
Androstendione and Estrogen
Some researchers suggest that androstenedione supplementation may increase serum estrogen levels in both males and females due to the conversion (aromatization) of the excess androstenedione and testosterone by aromatase (3,5). There is much evidence to suggest that aromatase activity occurs quite readily in the human body since both adipose tissue and muscle tissue reportedly have a large aromatizing capacity (12,13). High levels of estrogen have been linked to gynecomastia (breast development in men) (14), breast cancer (15), pancreatic cancer (16), and an increased risk of cardiovascular disease (17).
Some manufacturers of androstenedione supplements have included within their products certain compounds such as chrysin (flavone X) that are believed to temporarily inhibit the conversion of androstenedione and testosterone into estrogens. These compounds called "flavones" or "flavonoids" are derived from plants or may be synthetically produced. They appear to have a slightly higher affinity for aromatase (the enzyme, also known as estrogen synthase, that converts androstenedione and testosterone into estrogens) than the steroid substrates. The flavones are believed to either compete with androstenedione and testosterone for aromatase directly or alter the binding capacity of aromatase (18). However, studies that indicate that these flavones inhibit aromatase activity have only been conducted in vitro, and there is no evidence that this process can occur in humans.
Risks and
Disadvantages
|
| Recommendations Avoid this supplement (children and teenagers especially) until further investigation supports the safety as well as the efficacy of the supplement. |
Article by Richard Chiang 08/02/99
Revised 12/22/99
2.Smith AS, Fitch GK. Understanding Human Anatomy and Physiology. St. Paul, MN:West Publishing Company. 1993.
3. Myhal M, Wilson C. Muscle-building and fat-burning supplements. 13th annual Gatorade Sports Science Institute Scientific Conference; 1999 June 25-26: Chicago. Illinois: The Gatorade Company, 1999.
4. Blaquier J, Forchielli E, Dorfman RI. In vitro metabolism of androgens in whole human blood. Acta Endocrinol (Copenh). 1967;4:697-704.
5. King DS, Sharp RL, Vukovich MD, Brown GA, Reifenrath TA, Uhl
NL, Parsons KA. Effect of oral androstenedione on serum testosterone and adaptations to
resistance training in young men: a randomized controlled trial. JAMA. 1999
;21:2020-8.
6. Longcope C, Kato T, Horton R. Conversion of blood androgens to estrogens in normal adult men and women. J Clin Invest.1969;12:2191-201.
7. Bartsch W, Klein H, Schiemann U, Bauer HW, Voigt KD. Enzymes of androgen formation and degradation in the human prostate. Ann N Y Acad Sci. 1990;595:53-66.
8. Mahesh VB, Greenblatt RB. The in vivo conversion of dehydroepiandrosterone and androstenedione to testosterone in the human. Acta Endocrinol. 1962;41:400-6.
9. Savard K, Gut M, Dorfman RI, Gabrilove JL, Soffer LJ. Formation of androgens by human arrhenoblastoma tissue in vitro. J Clin Endocrinol. 1961;21:165-173.
10. Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;1:1-7.
11. Hacker R, Mattern C, inventors; Arrowdeen Ltd, assignee. German patent DE 42 14953 A1. 1995.
12. Forney JP, Milewich L, Chen GT, Garlock JL, Schwarz BE, Edman CD, MacDonald PC. Aromatization of androstenedione to estrone by human adipose tissue in vitro. Correlation with adipose tissue mass, age, and endometrial neoplasia. J Clin Endocrinol Metab. 1981;53:192-9.
13. Matsumine H, Hirato K, Yanaihara T, Tamada T, Yoshida M. Aromatization by skeletal muscle. J Clin Endocrinol Metab. 1986;63:717-20.
14. Lewin ML. Gynecomastia: the hypertrophy of the male breast. J Clin Endocrinol. 1941;1:511-514.
15. Cauley JA, Lucas FL, Kuller LH, Stone K, Browner W, Cummings SR. Elevated serum estradiol and testosterone concentrations are associated with a high risk for breast cancer. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 1999;130:270-7.
16. Fyssas I, Syrigos KN, Konstandoulakis MM, Papadopoulos S, Milingos N, Anapliotou M, Waxman J, Golematis BC. Sex hormone levels in the serum of patients with pancreatic adenocarcinoma. Horm Metab Res. 1997;3:115-8.
17. Phillips GB, Pinkernell BH, Jing TY. The association of hyperestrogenemia with coronary thrombosis in men. Arterioscler Thromb Vasc Biol. 1996;16:1383-7.
18. Kellis JT Jr, Vickery LE. Inhibition of human estrogen synthetase (aromatase) by flavones. Science. 1984;225:1032-4.
19. Barrett-Connor E, Garland C, McPhillips JB, Khaw KT, Wingard DL. A prospective, population-based study of androstenedione, estrogens, and prostatic cancer. Cancer Res. 1990;50:169-73.
20. Fernandez-del Castillo C, Robles-Diaz G, Diaz-Sanchez V, Altamirano A. Pancreatic cancer and androgen metabolism: high androstenedione and low testosterone serum levels. Pancreas. 1990;5:515-8.
21. United States Olympic Committee. Drug Education Control. http://www.usoc.org.html Accessed December 26, 1998.
22. Kuipers H, Wijnen JA, Hartgens F, Willems SM. Influence of anabolic
steroids on body composition, blood pressure, lipid profile and liver functions in body
builders. Int J Sports Med. 1991;4:413-8.
23. Kris-Etherton P, Burns J. Cardiovascular Nutrition. The American Dietetic Association. 1998
24. Rosenbloom C. Androstenedione: its potential safety concerns. Scan's Pulse. 1999;18:4-6.
25. Schnirring L. Androstenedione et al: nonprescription steroids. The Physician and Sportsmedicine. 1998;26:1-6.
26. Yesalis C. Medical, legal, and societal implications of androstenedione use. JAMA. 1999;281:2043-2044.