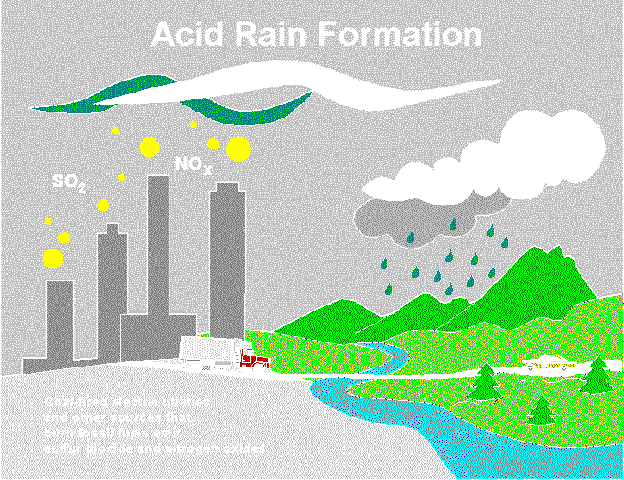
What causes acid rain?Acid rain is a result of a mixture of coal and oil being burned by power plants, factories and industries, and emissions being sent out by gas and diesel burning vehicles and engines. Acid rain is formed when rain falls through polluted air and dissolves chemicals that are present in the air such as sulfur oxides and nitrogen oxides. When they are wet, they become acids and fall to the earth, causing .
There are also a few natural causes of acid rain. One of these is volcanic eruptions. This releases crbon dioxide into the atmosphere which can form carbonic acid. Another possible natural cause of acid rain is the carbonic acid that is already in the atmosphere. However, it is hard to find any information on natural causes of acid rain. Most people like to focus on the things that man do to cause acid rain. |

Where does it effect?Most of the acid rain that falls in North America falls to the east of the Mississippi River. This is due first, to the heavy industry that is in the eastern and mid-western United States, and second, the fact that prevailing wind patterns flow from west to east, so pollutants are more likely to fall over the northeastern part of the continent.
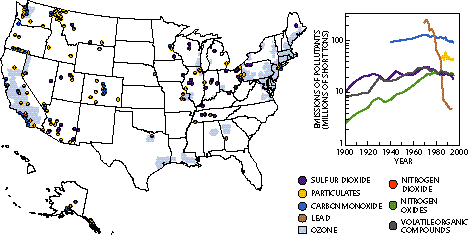
The EPA has focused on air concentrations of six pollutants: ozone, nitrogen dioxide, sulfur dioxide, carbon monoxide, particulates (soot) and lead. (The concern here is ground-level ozone, not ozone in the stratosphere, which blocks ultraviolet rays.) To see the human effects, check other effects of acid rain. |