|
Membrane associated thermosynthesis: MTS |
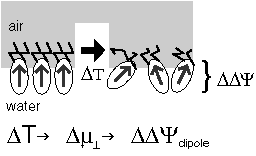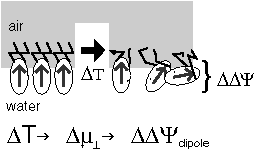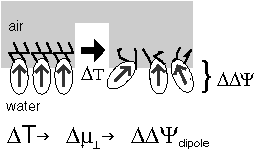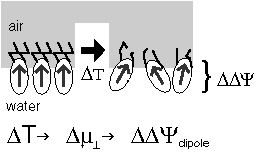
|
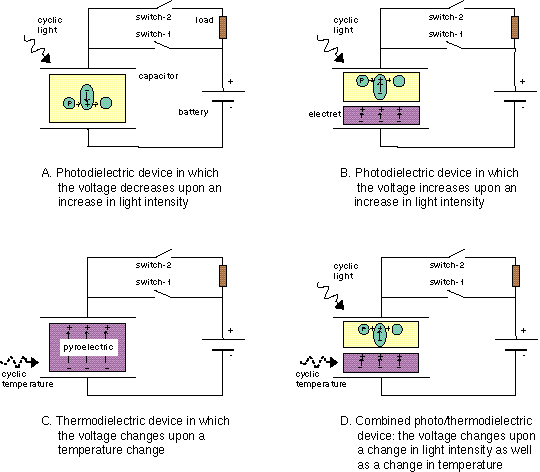
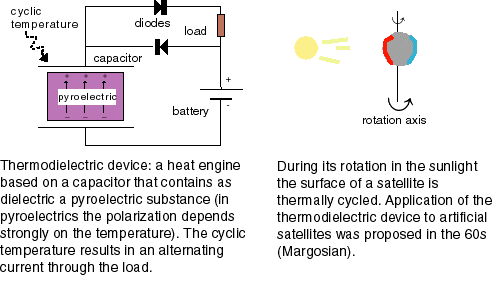
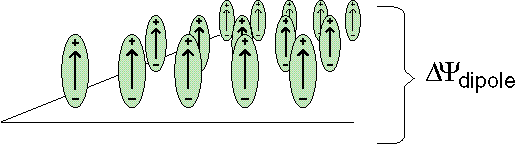
The voltage across a monolayer may vary with the temperature. At a certain temperature the variation may be very strong. Such strong variation is considered to be evidence of a phase transition in the monolayer. Upon the transition the disorder in the layer increases, and the dipole potential decreases. Only the average component of the dipole moment, µ, normal to the surface, µ ⊥ , contributes to the dipole potential. During the phase transition this average normal component decreases. |
|
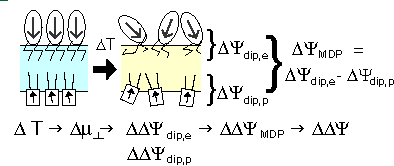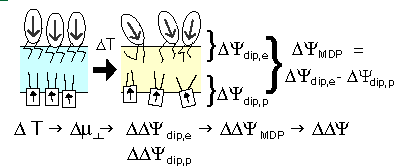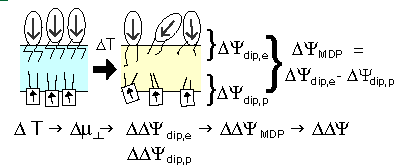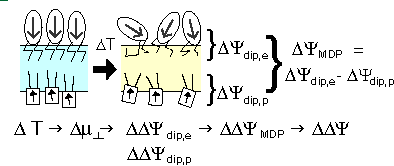
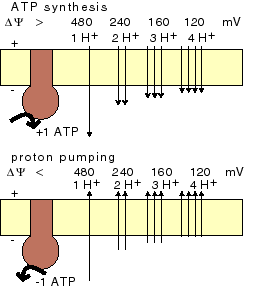
Consider first the relation between membrane potential and H+/ATP ratio. When the ratio is 1, it takes about 480 mV to synthesize ATP; is the ratio 2, it takes 480 / 2 = 240 mV to synthesize ATP, more generally, at ratio n it takes 480 / n mV. Conversely, protons can be pumped using ATP as long as the membrane voltage is lower than 480 mV (assuming H+/ATP ratio, or 'mode', 1), or 240 mV (mode 2), or, more generally, 480 / m mV (mode m). |
|
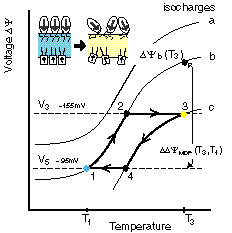
Cyclic variations of membrane potential during a thermal cycle, and associated changes in membrane potential due to voltage-activated ATP synthesis and proton pumping with different H+/ATP modes during MTS. |
|
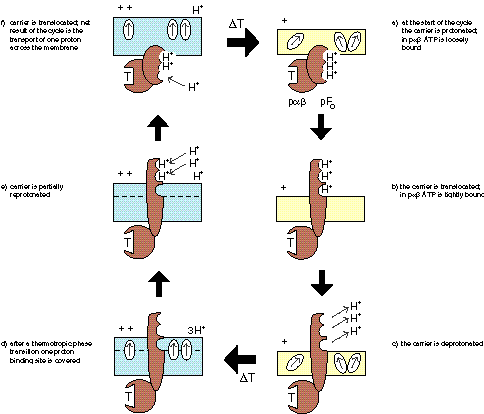
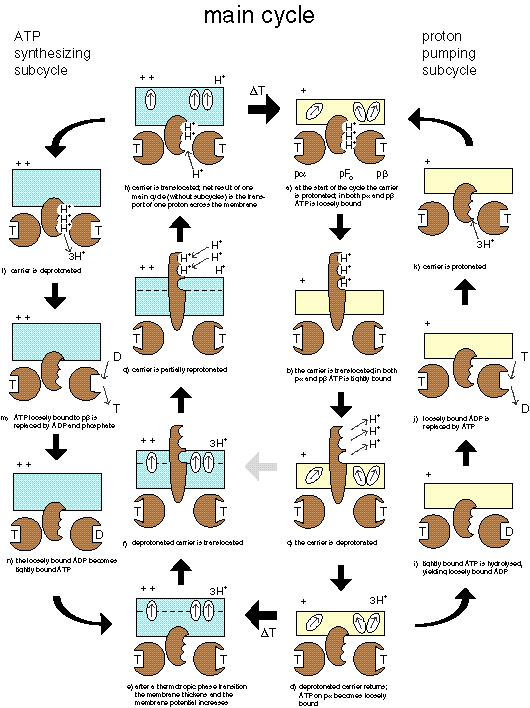
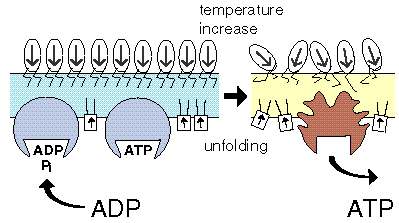
The evolution of ATPsynthase starts with a pF1 that is bound to a membrane that undergoes a thermotropic phase transition. Since the temperature interval required for a transition decreases with size, the advantage for the pF1 would be a smaller temperature interval for PTS to occur. The name pαβ is used to indicate that it may concern a progenitor of the α and β subunits present in contemporary pF1. |