In early development of several organs including pancreas, lung, submandibular gland, kidney and mammary gland, a bulbous epithelial bud generates a few lobules by forming clefts. Early morphogenesis of mouse submandibular gland provides an excellent model for the formation of epithelial lobules (Reviewed in Nakanishi and Ishii, 1989). In case of submandibular gland development, after repeated branching of the lobules, the shape of epithelium becomes like a bunch of grapes (See my simulations in Fig.6). This instance well exemplifies the general state of the problem: there is quite a wealth of cellular and biochemical information which gives strong indications of the main players in the game of morphogenesis.
Pattern Formation. It has been well established that glandular morphogenesis is under the influence of the associated mesenchymal cells. The mesenchymal cells is thought to surround young epithelial bulbs contacting with epithelial cells at outer surface of the bulb, at epithelia-mesenchyme interface. In fact, signals provided by the mesenchyme cells seems to be instructive (Gumbiner, 1992). The dominant feature is apparently establishment of non-uniform distributions of biochemical substances along the outer boundary of the developing multilobate form (Nakanishi and Ishii, 1989). Particularly, collagen III works as a key substance for cleft initiation in the morphogenesis of submandibular epithelium. The change in distribution of collagen III from even to uneven is thought to be critical for cleft initiation (Nakanishi et al., 1988).
At the beginning, mesenchymal cells surrounding young epithelial lobule secrete collagen at mesenchyme-epithelia interface and randomly move along outer surface of the lobule. Then, some mesenchyme cells begin to move in the same direction under the influence of interstitial collagen. The extracellular matrix is deformed by the traction forces of the cells, and in turn this induced anisotropy affects the cell movements. Initial irregularities in cell distributions in turn condense collagen bundles, while conditioning following condensation of the cells. Finally these groups of mesenchymal cells encircle the lobular surface generating and maintaining cyto-chemical pattern at the interface. This word picture from Nakanishi and Ishii (1989) has excellent mathematical description. I mean known model of Oster with co-workers (1983) describing macroscopic behavior of motile cells migrating within an elastic medium.
"Sensors" to the Epithelial Lobule Curvature. Nogawa's (1983) experiments demonstrated that each lobe of the submandibulary gland epithelium becomes smaller with progress of the development (from radius in limits 120-180 micrometers to radius in limits 40-100 micrometers). His studies strongly suggest that submandibular mesenchyme causes the epithelium to branch by increasing curvature of epithelial surface. As Nogawa asked, by what mechanism would submandibular mesenchime determine the curvature of epithelial surface? He assumed that studies on contact guidance by Dunn and Heath (1976) and Fisher and Tickle (1981) may suggest a possible mechanism. Namely, fibroblastic cells cannot hold firmly enough to a substratum whose curvature is large than a critical curvature and their microfilament bundles play an important role in this cell behavior. The cells is assumed to "sense" the geometry of the substratum by deformation of microfilament bundles. By the way, the curvature sensitivity threshold for fibroblast cells tested by Fisher and Tickle (1981), ranges in 100 to 200 micrometers of curvature radius, when substratum was fiber glass. (Compare these values with the radii of lobes!)
Pattern-Form Interplay. Comparative observations of the branching and elongating morphogenesis allow Nogawa (1983) to notice that the curvature of branching type epithelium becomes large with progress of the development, while that of elongating-type epithelium was nearly constant during of the elongation. This may suggest that "branching" can be regarded as increase in epithelial curvature.
Submandibular mesenchyme cells may have stage-dependent increasing critical curvature, and they may be able to affect the epithelial morphogenesis only when they can hold the epithelial surface. Hence, initially epithelial rudiment has view of a bulb until mesenchyme cells achieve critical curvature sensitivity and firmly hold the epithelial surface (Fig.6).
a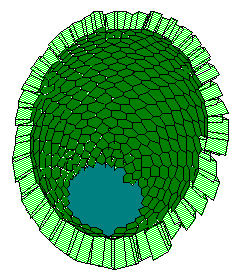 b
b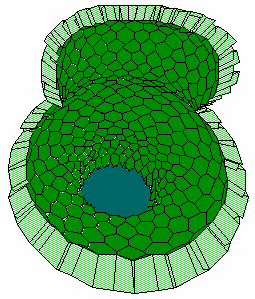 c
c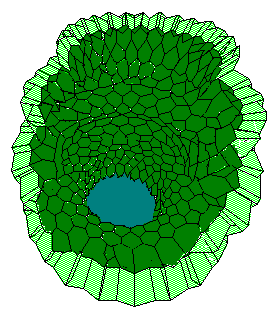 d
d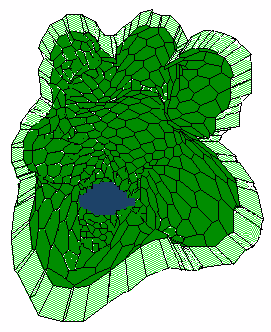
This follows pattern formation on the epithelia-mesenchyme interface, leading to uneven distribution of collagen III, particularly, at the outer surface of the bulb. In turn, the pattern initiates clefting process. As a result, the bulb will subdivide into two lobes. In turn, increasing of the epithelial surface curvature will lead to detachment of mesenchyme cells out of epithelium surface. This apparently abrupts deepening of interlabular cleft. With time, the critical curvature sensitivity increases and the surrounding mesenchyme cells again firmly attach to epithelia surface. And events will repeat.
On the contrary, when the mesenchyme-determined curvature is constant during development, the epithelium will form a cylindrical stalk without branching. If this consideration is added to the curvature-increasing model, the model will design various patterns of branching morphogenesis.