Acid-Base Titrations (Titration Curve)
The following sample data models the titration of a weak acid with a strong base.
| Volume Of Base (mL) | pH |
Volume Of Base (mL) |
pH |
|---|---|---|---|
1.00 |
3.15 |
15.00 |
4.90 |
2.00 |
3.24 |
16.00 |
5.20 |
3.00 |
3.39 |
16.50 |
5.40 |
4.00 |
3.54 |
17.00 |
5.60 |
5.00 |
3.63 |
17.50 |
5.95 |
6.00 |
3.78 |
18.00 |
6.60 |
7.00 |
3.85 |
18.50 |
7.30 |
8.00 |
3.98 |
18.70 |
7.60 |
9.00 |
4.11 |
18.90 |
8.15 |
10.00 |
4.20 |
19.10 |
9.95 |
10.50 |
4.26 |
19.30 |
10.50 |
11.00 |
4.31 |
20.50 |
10.90 |
11.50 |
4.39 |
21.00 |
11.80 |
12.00 |
4.47 |
21.50 |
12.20 |
13.00 |
4.60 |
22.50 |
12.30 |
14.00 |
4.75 |
23.50 |
12.35 |
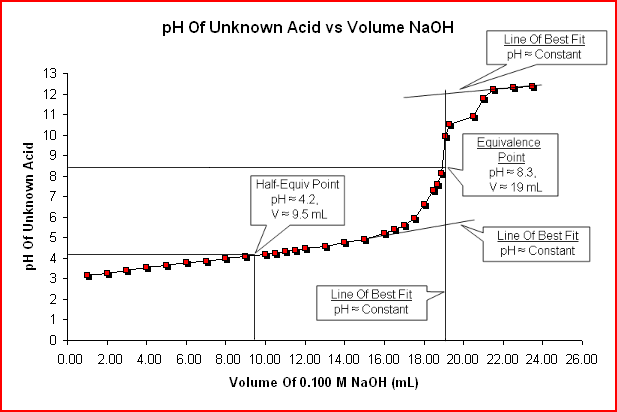
The titration curve below, pH vs Titrant Volume, represents the sample titration data.