#1
Copolymer-1 In The Treatment
Of Multiple Sclerosis
Boyden KM
J NeuroSci Nurs 1998 Apr; 30(2): 135-139
PMID# 9642622; UI# 98306590
Abstract
Recent research in the treatment of Multiple Sclerosis (MS) has yielded new therapies. Specifically, Copolymer-1, a mixture of synthetic polypeptides composed of four Amino Acids has been effective in reducing relapse rates and disability in patients with Relapsing/Remitting MS.
In a two-year multicenter, randomized, double-blind, placebo-controlled trial of 251 patients, Copolymer-1 was shown to reduce relapses by an average of 29% when compared with placebo.
Sustained disability was also slightly reduced in the Copolymer-1-treated group. The results of the clinical trial indicate that Copolymer-1 positively alters the course of Relapsing/Remitting MS.
With the present availability of Copolymer-1, nurses are challenged to maintain current knowledge of nursing implications, interventions, patient education and goals for treatment.
#2
Promiscuous Binding Of Synthetic Copolymer-1 To Purified HLA-DR Molecules
Fridkis-Hareli M, Strominger JL
J Immunol 1998 May 1; 160(9): 4386-4397
Harvard University, Dept of Molecular and Cellular Biology, Cambridge, MA 02138, USA
PMID# 9574543; UI# 98233687
Abstract
Copolymer-1 (Cop-1) is a random synthetic Amino Acid Copolymer of L-Alanine, L-Glutamic Acid, L-Lysine, and L-Tyrosine, effective both in suppression of Experimental Allergic EncephaloMyelitis and in the treatment of Relapsing forms of Multiple Sclerosis.
Cop-1 binds promiscuously and very efficiently to living APCs of various HLA haplotypes.
In the present study, a substantial part of the whole mixture of random PolyPeptides that compose Cop-1 was shown to bind to purified human HLA-DR1, DR2, and DR4 with high affinity in a temperature- and time (and, in the case of DR4, pH)-dependent manner, and was competitively inhibited by DR-restricted Peptides , but not by peptide derivatives that bind with low affinity.
Bacterial SuperAntigens inhibited Cop-1 binding only at very high concentrations. The formation of the Cop-1-DR1 complex was also shown by SDS-PAGE.
These findings represent the first direct evidence for interactions of Cop-1 with purified DR molecules, and suggest that its effectiveness in Experimental Allergic EncephaloMyelitis and Multiple Sclerosis may be directly related to its binding in the groove of HLA-DR proteins.
#3
Effect Of Copolymer-1 On Serial Gadolinium-Enhanced MRI In Relapsing/Remitting Multiple Sclerosis
Mancardi GL, Sardanelli F, Parodi RC, Melani E, Capello E, Inglese M, Ferrari A, Sormani MP, Ottonello C, Levrero F, Uccelli A, Bruzzi P
Neurology 1998 Apr; 50(4): 1127-1133
Univ of Genoa, Dept of Neurological Sciences, Italy
PMID# 9566406; UI# 98226057
Abstract
We examined the effect of Copolymer-1 (Cop-1) on Magnetic Resonance (MR) imaging changes in 10 patients with Relapsing/Remitting Multiple Sclerosis (RRMS).
Monthly Gadolinium (Gd)-enhanced MR imaging was performed for 9 to 27 months in the pretreatment period followed by 10 to 14 additional months during Cop-1 treatment. MR images were evaluated by two radiologists (F.S. and R.C.P.) masked to the scan date.
We found a 57% decrease in the frequency of new Gd-enhancing lesions and in the mean area/month of new Gd-enhancing lesions in the Cop-1 treatment period compared with the pretreatment period (0.92 versus 2.20 lesions per month and 22 mm2 versus 43 mm2 area/month; p = 0.1, Wilcoxon signed rank test).
Percentage change in lesion load area on T2-weighted images showed a decrease in the accumulation of lesion area during treatment, which was significant for the patient group with a longer pretreatment period (p = 0.05, Friedman test).
These results demonstrate a reduction in the number of new Gd-enhancing lesions and in the lesion load during Cop-1 treatment compared with the preceding period without therapy and are suggestive of an effect of Cop-1 on MR abnormalities observed in Multiple Sclerosis.
#4
Current Immunotherapy in Multiple Sclerosis
Bashir K, Whitaker JN
Immunol Cell Biol 1998 Feb; 76(1): 55-64
Univ of Alabama at BirminghamDept of Neurology, Birmingham, Alabama 35233-7340, USA
PMID# 9553777; UI# 98214393
Abstract
The underlying PathoPhysiology of Multiple Sclerosis is presumed to be AutoImmune in nature. Attempts to find an effective treatment for this common disease of the Central Nervous System have primarily focused on Immune-mediated therapies, both ImmunoSuppressive and ImmunoModulatory.
The wide variety of Immunological abnormalities detected in Multiple Sclerosis and its animal model, Experimental Allergic EncephaloMyelitis, has prompted the testing of a diverse array of drugs to be used for treatment.
Recent successes in the treatment of Relapsing/Remitting Multiple Sclerosis with Interferon-ß and Glatiramer Acetate have renewed interest in and raised expectations for the effective control of this Neurological Disorder.
Improved methodology in clinical trials, the development of surrogate markers and the availability of novel therapies bode well for more rapid advances.
#5
Glatiramer Acetate Blocks The Activation Of THP-1 Cells By Interferon-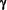
Li Q, Milo R, Panitch H, Swoveland P, Bever CT Jr
Eur J Pharmacol 1998 Jan 26; 342(2-3): 303-310
Univ of Maryland School of Medicine, Dept of Neurology, Baltimore, MD 21201, USA
PMID# 9548401; UI# 98208179
Abstract
Glatiramer Acetate (previously known as Copolymer-1) is a synthetic copolymer of four amino acids that has been approved for use in the treatment of Multiple Sclerosis.
It has been shown to suppress Myelin Antigen specific T-Cell activation by competing with these Antigens at the Major Histocompatibility Complex Class II binding site and by inducing Antigen specific Suppressor T-Cells.
In this study we investigated the effects of Glatiramer Acetate on the human Monocytic cell line, THP-1, activated by LipoPolySaccharide and Interferon-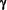 as a model for Macrophages. as a model for Macrophages.
At non-toxic concentrations of Glatiramer Acetate there were dose dependent reductions in the percentage of cells expressing human Leukocyte DR and DQ Antigen as well as in mean fluorescence intensity by flow cytometry.
Production of Tumor Necrosis Factor-alpha and the lysosomal cysteine proteinase cathepsin B were markedly inhibited, but production of InterLeukin-1 increased.
These results suggest that Glatiramer Acetate might alter Macrophage effector function and suggest that further studies in human Monocytes and Macrophages are warranted.
#6
Extended Use Of Glatiramer Acetate (Copaxone) Is Well Tolerated And Maintains Its Clinical Effect On Multiple Sclerosis Relapse Rate And Degree Of Disability. Copolymer-1 Multiple Sclerosis Study Group
Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, Myers LW, Panitch HS, Rose JW, Schiffer RB, Vollmer T, Weiner LP, Wolinsky JS
Neurology 1998 Mar; 50(3): 701-708
Univ of Maryland, Dept of Neurology, Baltimore 21201, USA
PMID# 9521260; UI# 98180556
Abstract
When 251 Relapsing/Remitting patients with Multiple Sclerosis were randomized to receive daily subcutaneous injections of Glatiramer Acetate, previously called Copolymer-1 (Copaxone; n = 125) or placebo (n = 126) for 24 months, there were no laboratory abnormalities associated with Glatiramer Acetate treatment and it was well tolerated with few side effects.
Patients receiving Glatiramer Acetate had significantly fewer relapses and were more likely to be Neurologically improved, whereas those receiving placebo were more likely to worsen.
This study was extended for 1 to 11 months (mean of 5.2 months for the Glatiramer Acetate group and 5.9 months for the placebo group). The blinding and study conditions used during the core 24-month study were unchanged throughout the extension.
The results of this extension study confirm the excellent tolerance and safety profile of Glatiramer Acetate for injection. The clinical benefit of Glatiramer Acetate for both the relapse rate and for Neurologic Disability was sustained at the end of the extension trial.
#7
Emerging Treatments In Multiple Sclerosis
Schluep M, Bogousslavsky J
Eur Neurol 1997; 38(3): 216-221
Centre Hospitalier Universitaire Vaudois, Service de Neurologie, Lausanne, Suisse
PMID# 9363834; UI# 98028464
Abstract
Currently used and developing therapies for Multiple Sclerosis (MS) are dealing with the treatment of acute exacerbations, the prevention of relapses and of the progression of Neurological Handicap, the treatment of symptoms, and the repair of DeMyelinated lesions.
New insights into the treatment of acute relapses as well as recent ImmunoModulatory and ImmunoSuppressive agents tested in MS are exposed in this brief review.
Interferon-ß (1a and 1b), Copolymer-1 and new ImmunoSuppressive therapies are particularly discussed with emphasis on their potential to alter the course of MS.
#8
Management Of Relapsing/Remitting Multiple Sclerosis With Copolymer-1 (Copaxone)
Johnson KP
Mult Scler 1996 Jul; 1(6): 325-326
Univ of Maryland Hospital, Dept of Neurology, Baltimore 21201, USA
PMID# 9345409; UI# 98005269
Abstract
Copolymer-I (Copaxone) was evaluated in a multicenter, placebo-controlled, double-blind trial at 11 universities.
Two hundred and fifty-one Relapsing/Remitting ambulatory MS patients were randomized to receive 20 mg of Copolymer-I or placebo by daily subcutaneous injection for approximately 30 months.
At conclusion, the Copolymer-I group had 32% fewer relapses (P = 0.002) and significantly more were relapse-free (P = 0.035).
Significantly, more patients were receiving Copolymer-I had improved during the study, while more patients on placebo showed Neurological decline (P = 0.001). There were few side effects and no drug related laboratory abnormalities.
Copolymer-I is being considered by North American and European regulatory agencies for approval as commercially available agent for the control of Multiple Sclerosis.
#9
Copolymer-1 Induces T-Cells Of The T-Helper Type 2 that Crossreact With Myelin Basic Protein And Suppress Experimental AutoImmune EncephaloMyelitis
Aharoni R, Teitelbaum D, Sela M, Arnon R
Proc Natl Acad Sci USA 1997 Sep 30; 94(20): 10821-10826
The Weizmann Institute of Science, Dept of Immunology, Rehovot 76100, Israel
PMID# 9380718; UI# 98021452
Abstract
The synthetic Amino Acid copolymer Copolymer-1 (Cop-1) suppresses Experimental AutoImmune EncephaloMyelitis (EAE) and is beneficial in Multiple Sclerosis.
To further understand Cop-1 suppressive activity, we studied the Cytokine secretion profile of various Cop-1-induced T-Cell lines and clones.
Unlike T-Cell lines induced by Myelin Basic Protein (MBP), which secreted either T-Cell helper type 1 (Th1) or both Th1 and Th2 Cytokines, the T-Cell lines/clones induced by Cop-1 showed a progressively polarized development toward the Th2 pathway, until they completely lost the ability to secrete Th1 Cytokines.
Our findings indicate that the polarization of the Cop-1-induced lines did not result from the Immunization vehicle or the in vitro growing conditions, but rather from the tendency of Cop-1 to preferentially induce a Th2 response.
The response of all of the Cop-1 specific lines/clones, which were originated in the (SJL/JxBALB/c)F1 hybrids, was restricted to the BALB/c parental haplotype. Even though the Cop-1-induced T-Cells had not been exposed to the AutoAntigen MBP, they crossreacted with MBP by secretion of InterLeukin (IL)-4, IL-6, and IL-10.
Administration of these T-Cells in vivo resulted in suppression of EAE induced by whole mouse spinal cord homogenate, in which several autoAntigens may be involved. Secretion of anti-inflammatory Cytokines by Cop-1-induced Suppressor Cells, in response to either Cop-1 or MBP, may explain the therapeutic effect of Cop-1 in EAE and in Multiple Sclerosis.
#10
PathoGenesis And Therapy Of Multiple Sclerosis
Nouza K, Krejcova H
Bratisl Lek Listy 1997 Apr; 98(4): 199-203
Ustav pro peci o matku a dite v Praze
PMID# 9264826; UI# 97365314
Abstract
Multiple Sclerosis (MS) is one of the most frequent serious Neurologic Diseases. Etiologically, MS involves Genetic, Viral and other factors.
The key Pathogenic mechanisms reside in the AutoImmune reaction of activated CD4+ T-Lymphocytes crossing the HaematoEncephalic Barrier and attacking different Epitopes of the Basic Protein and ProteoLipid of Myelin sheaths.
The damaging reaction involving activated Macrophages, destructive inflammatory Cytokines and toxic radicals leads to the development of disseminated plaques.
The passage of AutoImmune T-Lymphocytes to the Brain tissue is facilitated by overexpression of Adhesion Molecules on Endothelial Cells, Neural Cells and Immunocytes.
The diagnosis of MS is based on characteristic changes in the blood and liquor, and on the results of modern methods, especially (Gadolinium enhanced) MRI.
The "classical" treatment is based on GluCorticoSteroids, Azathioprine, Cyclophosphamide and other chemicals which are not fully satisfactory and are accompanied by serious side-effects. Therefore, attention will be paid to three prospective biological methods of treatment:
1) peroral application of bovine Myelin, its fractions, and synthetic Copolymer-1, aimed at the restoration of Immune tolerance;
2) injections of natural and recombinant Interferon-ß, interfering with the Pathogenic IFN-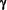 and other Cytokines; and other Cytokines;
3) systemic enzyme therapy (residing in peroral application of combinations of animal and herbal hydrolytic enzymes), which modulates Adhesion Molecules and suppresses the activation of AutoImmune T-Lymphocytes.
The chief results of clinical studies with respect to the effectiveness and safety of these therapeutic methods, will be summarized. (Ref. 34.)
#11
Current And Investigational Therapies Used To Alter The Course Of Disease In Multiple Sclerosis
Miller A
South Med J 1997 Apr; 90(4): 367-375
Maimonides Medical Center, Division of Neurology, Brooklyn, NY 11219, USA
PMID# 9114824; UI# 97269926
Abstract
Extensive research is under way to develop PharmacoTherapeutic agents that will prevent the exacerbations and the progression of Neurologic Disability associated with Multiple Sclerosis (MS).
The most intensive research strategy has involved agents intended to limit DeMyelination by reducing inflammation and modifying the Immune response.
In this category are Interferon-ß-1b, the first compound approved for use outside of clinical trials; Interferon-ß-1a; and Copolymer-1.
Experimental agents include other Interferons, Methotrexate, Linomide, MonoClonal AntiBodies, T-Cell receptor peptides, and 2-Chlorodeoxyadenosine.
Although they have been used effectively to treat exacerbations of MS, CorticoSteroids and CorticoTropin are now under evaluation as disease-modifying agents.
A second strategy, enhancing ReMyelination by limiting DeMyelination and Oligodendrocyte injury, is represented by protein growth factors.
A third therapeutic approach, improving conduction in DeMyelinated fibers, is represented by the Potassium Channel Blockers 4-Aminopyridine and 3,4-DiAminoPyridine.
#12
Modulation Of The Immune Response In Multiple Sclerosis: Production Of MonoClonal AntiBodies Specific To HLA/Myelin Basic Protein
Puri J, Arnon R, Gurevich E, Teitelbaum D
J Immunol 1997 Mar 1; 158(5): 2471-2476
The Weizmann Institute of Science, Dept of Immunology, Rehovot, Israel
PMID# 9036999; UI# 97188414
Abstract
MonoClonal Abs to the complex formed between human MHC Class II molecules (DR7 and DRw11) and Myelin Basic Protein (MBP) were produced.
The specificity of these Abs was established by both FACS analysis and complement-mediated cytotoxicity of MBP- or OVA-pulsed human APC of the same or of different DR restriction.
These Abs bound to and lysed only MBP-pulsed human APC of the same DR restriction (DR7 or DRw11) but not to APC of different DR restriction or pulsed with a different Ag (OVA).
The physiologic role of these Abs was further investigated. They blocked the in vitro proliferative response to MBP-specific T-Cell clones isolated from Multiple Sclerosis patients in an Antigen-specific and DR-restricted manner.
However, the Abs did not affect the response of MBP-specific T-Cell clones of other DR restriction nor did they interfere with the response to other Ags (purified protein derivative or Copolymer-1) presented on APC with the same DR restriction.
These Abs may be useful for treating Multiple Sclerosis in which reactivity to MBP is implicated. Moreover, this approach may be extended to other AutoAntigens and their counterpart AutoImmune Diseases.
#13
Cop-1 As A Candidate Drug For Multiple Sclerosis
Teitelbaum D, Arnon R, Sela M
J Neural Transm Suppl 1997;49: 85-91
Weizmann Institute of Science, Dept of Immunology, Rehovot, Israel
PMID# 9266417; UI# 97411439
Abstract
Copolymer-1 (Cop-1), a synthetic copolymer of Amino Acids, is very effective in suppression of Experimental AutoImmune EncephaloMyelitis (EAE), the animal model for Multiple Sclerosis (MS).
Cop-1 was found incapable of inducing EAE, yet it suppressed EAE in a variety of animal species, including primates.
The Immunological cross-reaction between the Myelin Basic Protein (MBP) and Cop-1 serves as the basis for the suppressive activity of Cop-1 in EAE, by the induction of Antigen-specific suppressor cells and competition with MBP for binding to Major Histocompatibility Complex (MHC) molecules.
Clinical trials with Cop-1, both Phase II and Phase III, were performed in Relapsing/Remitting (E-R) patients. The latter, a two-year multicenter double blind trial with 251 participating patients was conducted at 11 leading medical centers in the USA.
It demonstrated a significant beneficial effect of Cop-1 in both diminishing the rate of exacerbations and improving the clinical status. The side effects of Cop-1 were only minimal. The cumulative results indicate that Cop-1 is a promising candidate drug for Multiple Sclerosis.
#14
Copolymer-1
From Basic Research To Clinical Application
Teitelbaum D, Arnon R, Sela M
Cell Mol Life Sci 1997 Jan; 53(1): 24-28
Weizmann Institute of Science, Dept of Immunology, Rehovot, Israel
PMID# 9117994; UI# 97257991
Abstract
Copolymer-1 (Cop-1) is a synthetic Amino Acid copolymer effective in suppression of Experimental Allergic EncephaloMyelitis (EAE).
The suppressive effect of Cop-1 in EAE is a general phenomenon and is not restricted to a particular species, disease type or EnCephalitogen used for EAE induction.
In phase III clinical trials Cop-1 was found to slow progression of disability and reduce the relapse rate in Exacerbating/Remitting Multiple Sclerosis (MS).
In vivo and in vitro studies suggest that the mechanism for Cop-1 activity in EAE and MS involves the binding of Cop-1 to Major Histocompatibility Complex Class II molecules as an initial step.
This binding results both in competition with Myelin Antigens for T-Cell activation and in induction of specific Suppressor Cells of the Th2 type. As an Antigen-specific intervention, Cop-1 has the advantage of reduced probability of long-term damage to the Immune system.
|