Abstract
To test the utility of Gene therapeutic approaches for the treatment of Pain.
A recombinant Herpes Simplex Virus, type 1, has been engineered to contain the cDNA for an Opioid Peptide Precursor, human PreproEnkephalin, under control of the human CytomegaloVirus Promoter.
This virus and a similar recombinant containing the Escherichia Coli lacZ Gene were applied to the abraded skin of the Dorsal hindpaw of mice.
After infection, the presence of 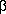 -galactosidase in Neuronal cell bodies of the relevant Spinal Ganglia (lacZ-containing virus) and of human ProEnkephalin (PreproEnkephalin-encoding virus).
-galactosidase in Neuronal cell bodies of the relevant Spinal Ganglia (lacZ-containing virus) and of human ProEnkephalin (PreproEnkephalin-encoding virus).
In the central terminals of these Neurons indicated appropriate Gene delivery and expression.
Baseline foot withdrawal responses to noxious radiant heat mediated by A and C fibers were similar in animals infected with ProEnkephalin-encoding and
and C fibers were similar in animals infected with ProEnkephalin-encoding and 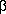 -galactosidase-encoding viruses.
-galactosidase-encoding viruses.
Sensitization of the foot withdrawal response after application of Capsaicin (C fibers) or Dimethyl Sulfoxide (A fibers).
fibers).
Observed in control animals was reduced or eliminated in animals infected with the ProEnkephalin-encoding virus for at least 7 weeks postinfection.
Hence, PreproEnkephalin cDNA delivery selectively blocked HyperAlgesia without disrupting baseline Sensory NeuroTransmission.
This blockade of sensitization was reversed by administration of the Opioid Antagonist Naloxone, apparently acting in the Spinal Cord.
The results demonstrate that the function of Sensory Neurons can be selectively altered by Viral delivery of a TransGene.
Because HyperAlgesic mechanisms may be important in establishing and maintaining NeuroPathic and other Chronic Pain states, this approach may be useful for treatment of Chronic Pain and HyperAlgesia in humans.
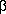 -galactosidase in Neuronal cell bodies of the relevant Spinal Ganglia (lacZ-containing virus) and of human ProEnkephalin (PreproEnkephalin-encoding virus).
-galactosidase in Neuronal cell bodies of the relevant Spinal Ganglia (lacZ-containing virus) and of human ProEnkephalin (PreproEnkephalin-encoding virus). and C fibers were similar in animals infected with ProEnkephalin-encoding and
and C fibers were similar in animals infected with ProEnkephalin-encoding and 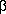 -galactosidase-encoding viruses.
-galactosidase-encoding viruses. fibers).
fibers).